ExCellerate Human T Cell Expansion Media, Xeno-Free Summary
Media for the ex vivo expansion and culture of human T cell lymphocytes under xeno-free conditions.Key Benefits
- Robust T cell expansion under serum-free and xeno-free conditions
- Compatible with a variety of T cell activation methods
- Supports T cell expansion from PBMCs or purified CD3+ T cells
Why Use ExCellerate Human T Cell Expansion Media?
T Lymphocytes (T Cells) are a critical component of the adaptive immune response. While dysregulation of T cell function and proliferation contributes to the etiology of many diseases, the ability of T cells to activate an immune response toward specific antigens is being harnessed as a powerful immunotherapy tool to combat cancer and other diseases. To facilitate discovery and preclinical research for T cell immunoregulation and therapy, robust platforms for ex vivo expansion and maintenance of T cells, including specialized serum-free or xeno-free media, are required.
ExCellerate Human T Cell Expansion Media is formulated and optimized for the ex vivo culture of human T lymphocytes in research applications. Unlike traditional serum-containing culture media, xeno-free media provides a stable culture and optimized environment, void of non-human animal-derived products, that facilitates the generation and expansion of T cells. The medium supports expansion of routine culture of human T cell lines or T cell clones and the stimulation of human peripheral blood lymphocytes.
Versatility of ExCellerate Human T Cell Expansion Media.
ExCellerate Human T Cell Expansion Media is optimized for T cell expansion when used in combination with Recombinant Human Cytokines (IL-2 or IL-7 and IL-15) and cell activation methods, including particle, bead-based, or plate-bound anti-CD3/anti-CD28 antibodies. The activation and cytokine/growth factor combinations used with this media should be optimized by application or experimental protocol.
Product Details
- 500 mL of ExCellerate Human T Cell Expansion Media
Data Examples
Fold Expansion of Human T Cells in ExCellerate Human T Cell Expansion Media. Primary human peripheral blood mononuclear cells (PBMCs) were cultured for 9 days in ExCellerate Human T Cell Expansion Media using activating beads and Recombinant Human IL-2 (R&D Systems, Catalog # 202-IL). Cell counts were performed to determine fold expansion compared to the Day 0 seeding density (0.25 x 106 cells/mL).
Phenotypic Analysis of Human T cells Expanded in Xeno-Free Human T Cell Expansion Media. Human PBMCs were cultured for 9 days in ExCellerate Human T Cell Expansion Media using activating beads and Recombinant Human IL-2 (R&D Systems, Catalog # 202-IL). Human T Cells were enriched during the culture as indicated via the expression of positive T cell markers, including Mouse anti-Human CD3 epsilon APC-conjugated Antibody (Catalog # FAB100A) and with a Mouse Anti-Human CD8 alpha PE-conjugated Antibody (Catalog # FAB1509).
ExCellerate Human T Cell Expansion Media Enhances T Cell Expansion Compared to Other Commercial Media. 2.5 x 106 primary human peripheral blood mononuclear cells (PBMCs) were cultured for 9 days using ExCellerate Human T Cell Expansion Media or other commercially available media (Supplier #1 or #2), in combination with Recombinant Human IL-2 GMP (Catalog # 202-GMP), Recombinant Human IL-15 (Catalog # 247-GMP), Recombinant Human IL-7 GMP (Catalog # 207-GMP). The total number of T cells (A) and CD8+ T cells (B) were determined at days 5 and 9. ExCellerate Human T Cell Expansion Media shows superior expansion rates compared to other commercial media.
Performance of GMP Cytokines in ExCellerate Human T Cell Expansion Media. Primary human peripheral blood mononuclear cells (PBMCs) were cultured for 12 days in ExCellerate Human T Cell Expansion Media or another commercially available T cell media (Supplier #1) containing combinations of Recombinant Human IL-2 GMP (Catalog # 202-GMP), Recombinant Human IL-15 (Catalog # 247-GMP), Recombinant Human IL-7 GMP (Catalog # 207-GMP). The total number of T cells were quantified at days 0, 5, 8, and 12. Under all GMP cytokine conditions, T cells cultured using ExCellerate Human T Cell Expansion Media showed superior expansion compared to Supplier #1 media.
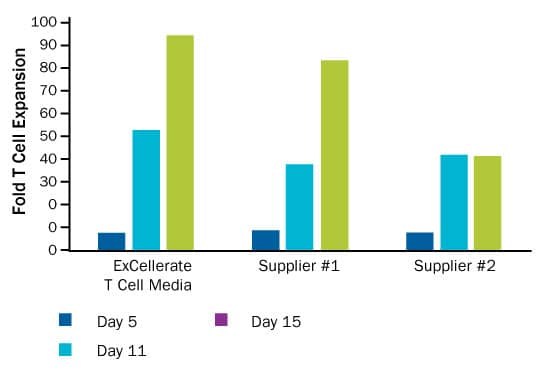
Superior Human T Cell Expansion Using ExCellerate Human T Cell Expansion Media and Plate-bound Anti-CD3 and Anti-CD28 Antibody Stimulation. Primary human peripheral blood mononuclear cells (PBMCs) were cultured for 15 days in ExCellerate Human T Cell Expansion Media or other commercially available media (Supplier #1 or #2), in combination with Recombinant Human IL-2 (R&D Systems, Catalog # 202-IL) and plate-bound Mouse Anti-Human CD3 epsilon Antibody (Catalog # MAB100) and Human CD28 Antibody (Catalog # AF-342-PB). Fold expansion was determined and cell counts were performed at days 5, 11, and 15 to determine fold expansion compared to the Day 0 seeding density. ExCellerate Human T Cell Expansion Media shows superior expansion rates compared to other commercial media.
Lower Exhaustion Phenotype of T Cells Cultured Using ExCellerate Human T Cell Expansion Media Compared to Other T Cell Media. Primary human peripheral blood mononuclear cells (PBMCs) were cultured (0.4 x 106 cells/mL) for 9 days using either ExCellerate Human T Cell Expansion Media or another commercially available T cell expansion media (Supplier 1). Both media were supplemented with Recombinant Human IL-2 (R&D Systems, Catalog # 202-IL) and activating microbeads. (A) At days 5, 7, and 9, T cells fold expansion was determined determined. (B) T cells were resuspended in their respective media (0.4 x 106 cells/mL), restimulated in culture with activating beads for an additional 6 days. At days 12 and 15 in culture, cells restimulated in ExCellerate Human T Cell Expansion Media showed continued expansion of T cells compared to the lower expansion rates of cell cultured in Supplier 1 media. (C) T cells were analyzed by flow cytometry for expression of PD-1, TIM3, LAG-1, and TIGIT, common surface markers of cell exhaustion. CD4+ T cells restimulated and cultured using ExCellerate Human T Cell Expansion Media showed low expression of cell exhaustion markers. Exhaustion markers on cells grown in Supplier 1 media show elevated expression levels.
Specifications
Product Datasheets
FAQs
No product specific FAQs exist for this product, however you may
View all Cell Culture Product FAQsReviews for ExCellerate Human T Cell Expansion Media, Xeno-Free
There are currently no reviews for this product. Be the first to review ExCellerate Human T Cell Expansion Media, Xeno-Free and earn rewards!
Have you used ExCellerate Human T Cell Expansion Media, Xeno-Free?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥1250 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image