MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit
MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit Summary
For the isolation of mouse regulatory T cells from a preparation of splenocytes.
Key Benefits
- Does not require specialized instrumentation
- Obtains isolated Treg cells by a combination of negative and positive selection
Why Isolate Regulatory T cells?
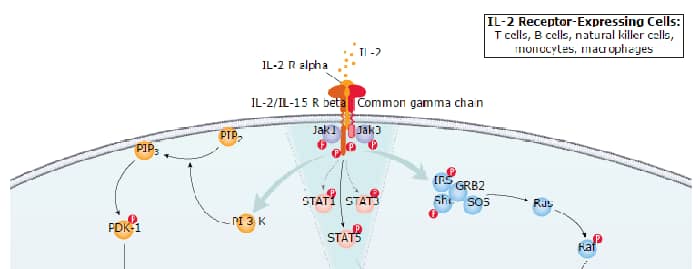
Regulatory T cells (Treg), are a minor subset of CD4+ T cells that can suppress the development and progression of immune reactions. They also play an important role in the balance between tolerance and autoimmunity. Treg cells are characterized by the expression of CD4 and CD25 on the cell surface and the intracellular transcription factor FoxP3. The isolation of regulatory T cells facilitates research into their critical role in immune system regulation.
Subsets and Phenotypes
Treg cells generated in the thymus are known as natural Treg (nTreg) or thymic Treg (tTreg). Alternatively, Treg cells can differentiate in the periphery from naïve CD4+ T cells. These cells are known as induced Treg (iTreg) or peripherally-derived Treg (pTreg). pTreg cells can further differentiate into subsets with variable expression of CCR4, CCR5, CCR6, CCR10, and CXCR3. Differential chemokine receptor expression enables these cells to migrate to specific tissues and inflammatory sites. Some populations of Treg cells do not express FoxP3 but retain an immune suppressive function, while other populations express FoxP3 but do not exhibit suppressive activity. Additional Treg molecules that may aid in the discrimination between Treg cell populations include the cell surface proteins PD-1, Neuropilin, CD73, and LRRC32/GARP and the transcription factor Helios. There are significant differences in the generation and function of Treg cells in mice and in humans.
Induction and Mechanism of Action
The expression of FoxP3 is integral to the in vivo development of Treg cells. The presence of TGF-beta, IL-2, and IL-10 in tissue culture supports Treg cell differentiation and expansion in vitro. Dendritic cells (DC) support Treg cell development and expansion, and Treg cells in turn regulate the differentiation of DC precursors. Treg cells limit T effector cell differentiation, proliferation, and activation, as well as humoral B cell responses. Mechanistically, FoxP3 directly blocks transcription of the pro-inflammatory cytokines IL-17 and IFN-gamma. Cell surface CTLA-4 and LAG3 and secreted IL-10, IL-35, and TGF-beta are additionally implicated in Treg cell function.
Treg in Disease
Treg cell-mediated immune suppression is important for restraining immune reactions, but this activity can be damaging in the context of disease. For example, Treg cell generation in response to oral antigens or intestinal microbes promotes the development of immune tolerance. In contrast, gut Treg cells can contribute to chronic inflammatory states such as colitis. In cancer, elevated numbers of Treg cells are found within tumors. This imbalance is a result of the actions of local dendritic cells that recruit tTreg and also expand and induce pTreg. Intratumoral Treg cells then contribute to the suppression of host anti-tumor immunity. The immune dysregulatory syndrome IPEX results from mutations in FoxP3, and IPEX patients suffer from a variety of autoimmune disorders.
References:
Abbas, A.K. et al. (2013) Nat. Immunol. 14:307.Schmitt, E.G. and C.B. Williams (2013) Front. Immunol. 4:152.
Adeegbe, D.O. and H. Nishikawa (2013) Front. Immunol. 4:190.
The MagCellect Mouse CD4+CD25+ Regulatory T Cell Isolation Kit (Catalog # MAGM208) contains the following reagents that allow for isolation of highly pure mouse Treg cells.
- MagCellect Mouse CD4+ T Cell Biotinylated Antibody Cocktail; 1 mL in PBS containing BSA and preservative
- MagCellect Streptavidin Ferrofluid; 1.5 mL in a solution containing BSA and preservative
- Anti-Mouse CD25 Biotinylated Antibody; 0.5 mL in a solution containing BSA and preservative
- MagCellect Plus Buffer (10X); 25 mL of a 10X concentrated buffer
*This kit contains sufficient reagents to process 1 x 109 total cells.
Stability and Storage
Store all reagents at 2 °C to 8 °C. DO NOT FREEZE.
Specifications
Product Datasheets
Scientific Data
 View Larger
View Larger
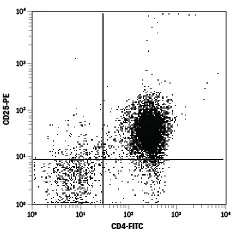
Enrichment of CD4+CD25+ Regulatory T cells from mouse splenocytes using the MagCellect Mouse CD4+CD25+ Regulatory T Cell Isolation Kit. In the first step, CD4+ T cells were isolated from splenocytes. In the second step, CD4+CD25+ T cells were isolated from the CD4+ cells recovered in Step 1. CD4+CD25+ T cells were stained using FITC-conjugated Rat Anti-Mouse CD4 Monoclonal Antibody (Catalog #FAB554F) and PE-conjugated Rat Anti-Mouse CD25 Monoclonal Antibody (Catalog # FAB2438P).
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, mouse CD4+ regulatory T cells can be isolated from PBMC using the following procedure:
Step 1
- Incubate the single-cell suspension of splenocytes with the MagCellect Mouse CD4+ T Cell Biotinylated Antibody Cocktail
- Add the MagCellect Streptavidin Ferrofluid
- Place the tube in the MagCellect Magnet
- Collect the CD4+ cells while undesired cells remain attracted to the magnet
Step 2
- Incubate the CD4+ T cell suspension with the MagCellect Anti-Mouse CD25 Biotinylated Antibody
- Add the MagCellect Streptavidin Ferrofluid
- Place the tube in the MagCellect Magnet
- While the tube is in the magnet, remove the unwanted cells from the tube
- Remove the tube from the magnet and resuspend the CD4+CD25+ T cells in buffer or media for counting
Reagents Supplied in the MagCellect Mouse CD4+CD25+ Regulatory T Cell Isolation Kit (Catalog # MAGM208)
- MagCellect Mouse CD4+ T Cell Biotinylated Antibody Cocktail; 1 mL in PBS containing BSA and preservative
- MagCellect Streptavidin Ferrofluid; 1.5 mL in a solution containing BSA and preservative
- Anti-Mouse CD25 Biotinylated Antibody; 0.5 mL in a solution containing BSA and preservative
- MagCellect Plus Buffer (10X); 25 mL of a 10X concentrated buffer
This kit contains sufficient reagents to process up to 1 x 109 total cells.
- MagCellect Magnet (Catalog # MAG997) or equivalent
- 12 x 75 mm (5 mL) or 17 x 100 mm (15 mL) polystyrene round bottom tubes
- 15 mL conical centrifuge tubes and benchtop centrifuge
- Sterile Pasteur pipettes or transfer pipettes
NOTE: Reaction incubations must be carried out at 2 to 8° C in a refrigerator and not in an ice bath to avoid excessively low temperatures that can slow the kinetics of the optimized reactions.
R&D Systems Protocol for the Magnetic Isolation of Mouse Regulatory T Cells
PBMC Preparation
Enrich for mononuclear cells by using a density gradient or any other method that provides a single cell suspension.
Wash the cells 2 times with excess PBS.
Centrifuge the cells for 10 minutes at 200 x g.

Decant the supernatant. If necessary, remove red blood cells using R&D Systems Mouse Erythrocyte Lysing Kit (Catalog # WL2000).
Resuspend the cells in a small volume of cold 1X MagCellect Plus Buffer.

Perform a cell count.
Adjust the cell concentration to 1 x 108 cells/mL with cold 1X MagCellect Plus Buffer.

Step 1 - Negative selection of CD4+ T cells
Transfer 2 x 108 cells (1.0 mL) into a 5-mL polystyrene tube.
Add 200 μL of MagCellect Mouse CD4+ T Cell Biotinylated Antibody Cocktail.
Mix the cell-antibody suspension and incubate at 2 °C to 8 °C for 15 minutes.

Add 250 μL of MagCellect Streptavidin Ferrofluid to the cell suspension.
Mix gently.
Incubate at 2 °C to 8 °C for 15 minutes.

Add 1.6 mL of cold 1X MagCellect Plus Buffer.
Mix gently.

Place the reaction tube in the MagCellect Magnet.
Incubate for 6 minutes at room temperature (18 °C to 25 °C).
Transfer the supernatant containing the CD4+ T cells into a new 5 mL tube.

Repeat the magnetic depletion with the new tube containing the recovered cells. The supernatant obtained at the end of this step is the final depleted cell fraction containing the desired enriched CD4+ T cells.

Step 2 - Positive selection for CD4+CD25+ T cells
Add 10 µL of MagCellect Mouse CD25+ T Cell Biotinylated Antibody Cocktail per 1 x 107 cells.
Mix the cell-antibody suspension and incubate at 2 °C to 8 °C for 15 minutes.

Add 250 µL of MagCellect Streptavidin Ferrofluid per 1 x 107 cells in the suspension.
Mix gently.
Incubate at 2 °C to 8 °C for 15 minutes.

Add cold 1X MagCellect Plus Buffer to reach a volume of 1.0 mL.
Mix gently.

Place the reaction tube in the MagCellect Magnet.
Incubate for 6 minutes at room temperature (18 °C to 25 °C).
Remove unwanted cell suspension from the tube.
Remove tube from the magnet and resuspend the CD4+CD25+ T cells in 1.0 mL cold 1X MagCellect buffer.

To complete the cell isolation procedure, repeat Step 2 with the resuspended cell fraction.
The cells are now ready for counting and further downstream applications. Mouse Treg cell populations isolated by this negative and positive selection protocol typically have a purity of 84-94%.

Technical Hints
- If sterile cells are required following the cell selection, the entire procedure should be carried out in a laminar flow hood to maintain sterile conditions. Use sterile equipment when pipetting reagents that will be reused at a later date.
- Avoid antibody capping on cell surfaces and non-specific cell tagging by working fast, keeping cells and solutions cold through the use of pre-cooled solutions and by adhering to the incubation times and temperatures specified in the protocol. Increased temperature and prolonged incubation times may lead to non-specific cell labeling thus lowering cell purity and yield.
- When processing different numbers of cells observe the following guidelines: keep antibody cocktail and ferrofluid incubation times and temperatures the same; keep the cell density at 10 x 107 cells/mL; add 10 μL of the antibody cocktail per 1 x 107 cells being processed; add 12.5 μL of Streptavidin Ferrofluid per 1 x 107 cells being processed.
- When processing 20 x 107 cells or fewer, use the 12 x 75 mm (5 mL) tubes with the MagCellect Magnet horizontally positioned to accommodate up to six 5 mL tubes. Do not process more than 20 x 107 cells in each 5 mL tube and do not exceed a total reaction volume of 3 mL in each tube. A reaction volume of 2 mL is recommended for processing 10 x 107 cells. A reaction volume of 1 mL is recommended when processing 5 x 107 or fewer cells. Reaction volume adjustments must be made using 1X MagCellect Buffer just prior to the magnetic separation step.
- When processing greater than 20 x 107 cells, use the 17 x 100 mm (15 mL) tubes with the MagCellect magnet vertically positioned to accommodate up to two 15 mL tubes. Do not process more than 60 x 107 cells in each 15 mL tube and do not exceed a total reaction volume of 9 mL in each tube. When using this larger tube, increase the reaction volume before the magnetic separation step according to the following formula: 3 mL for each 20 x 107 cells processed. Also increase the magnetic incubation time described in to 8 minutes. Reaction volume adjustments must be made using 1X MagCellect Buffer just prior to the magnetic separation step.
Citations for MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
13
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine
Authors: H Li, T Wen, T Wang, Y Ji, Y Shen, J Chen, H Xu, X Wu
Int J Mol Sci, 2020-01-08;21(2):. 2020-01-08
-
Critical Role of Regulatory T Cells in the Latency and Stress-Induced Reactivation of HSV-1
Authors: W Yu, S Geng, Y Suo, X Wei, Q Cai, B Wu, X Zhou, Y Shi, B Wang
Cell Rep, 2018-11-27;25(9):2379-2389.e3. 2018-11-27
-
Induced Regulatory T Cells Superimpose Their Suppressive Capacity with Effector T Cells in Lymph Nodes via Antigen-Specific S1p1-Dependent Egress Blockage
Authors: S Geng, Y Zhong, X Zhou, G Zhao, X Xie, Y Pei, H Liu, H Zhang, Y Shi, B Wang
Front Immunol, 2017-06-07;8(0):663. 2017-06-07
-
Early Pregnancy Factor Enhances the Generation and Function of CD4(+)CD25(+) Regulatory T Cells
Authors: Honghua Yuan
Tohoku J. Exp. Med., 2016-11-01;240(3):215-220. 2016-11-01
-
The C-Terminally Encoded, MHC Class II-Restricted T Cell Antigenicity of the Helicobacter pylori Virulence Factor CagA Promotes Gastric Preneoplasia.
Authors: Arnold IC, Hitzler I, Engler D, Oertli M, Agger EM, Muller A
J. Immunol., 2011-04-25;186(11):6165-72. 2011-04-25
-
Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice.
Authors: Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B
Endocrinology, 2010-09-15;151(11):5477-88. 2010-09-15
-
Enhancement of humoral and cellular responses to HBsAg DNA vaccination by immunization with praziquantel through inhibition TGF-beta/Smad2,3 signaling.
Authors: Zou Q, Zhong Y, Su H, Kang Y, Jin J, Liu Q, Geng S, Zhao G, Wang B
Vaccine, 2010-02-23;28(8):2032-8. 2010-02-23
-
An immunotherapeutic treatment against flea allergy dermatitis in cats by co-immunization of DNA and protein vaccines.
Authors: Jin J, Ding Z, Meng F, Liu Q, Ng T, Hu Y, Zhao G, Zhai B, Chu HJ, Wang B
Vaccine, 2010-02-23;28(8):1997-2004. 2010-02-23
-
Protective response against type 1 diabetes in nonobese diabetic mice after coimmunization with insulin and DNA encoding proinsulin.
Authors: Zhang W, Jin H, Hu Y, Yu Y, Li X, Ding Z, Kang Y, Wang B
Hum. Gene Ther., 2010-02-01;21(2):171-8. 2010-02-01
-
Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide.
Authors: Brahmachari S, Pahan K
J. Immunol., 2009-07-08;183(3):2045-58. 2009-07-08
-
Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity.
Authors: Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de Meester C, Bertrand L, Singh I, Chen Y, Viollet B, Giri S
Biochem. Biophys. Res. Commun., 2009-05-30;386(1):16-20. 2009-05-30
-
Induction of adaptive T regulatory cells that suppress the allergic response by coimmunization of DNA and protein vaccines.
Authors: Jin H, Kang Y, Zhao L, Xiao C, Hu Y, She R, Yu Y, Du X, Zhao G, Ng T, Chu HJ, Wang B
J. Immunol., 2008-04-15;180(8):5360-72. 2008-04-15
-
Co-inoculation of DNA and protein vaccines induces antigen-specific T cell suppression.
Authors: Zheng G, Geng S
Biochem. Biophys. Res. Commun., 2006-12-26;353(4):1034-9. 2006-12-26
FAQs
-
When using MacCellect Cell Isolation kits (MAGM*** or MAGH***), why is it important to lyse red blood cells before starting the cell isolation protocol?
It is important to remove red blood cells before the isolation protocol using MagCellect isolation kits because not doing so can cause a poorer yield of selected cells. Red blood cells become sticky during the incubation protocols and may interfere with the interactions needed for removal of unwanted populations.
-
What is the purity of the enriched CD4+ CD25+ Regulatory T cell population when using the MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit, Catalog # MAGM208?
The purity of CD4+ CD28+ T cells recovered using MAGM208 kit is >60%. This is using a starting population of resting T cells.
Reviews for MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit
Average Rating: 4 (Based on 1 Review)
Have you used MagCellect Mouse CD4+ CD25+ Regulatory T Cell Isolation Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: