Mouse DLL4 Antibody Summary
Ser28-Pro525
Accession # BAB18580
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
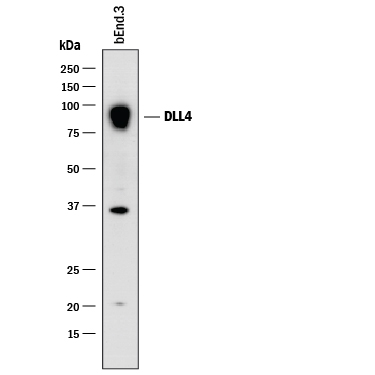 View Larger
View Larger
Detection of Mouse DLL4 by Western Blot. Western blot shows lysates of bEnd.3 mouse endothelioma cell line. PVDF membrane was probed with 2 µg/mL of Goat Anti-Mouse DLL4 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1389) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for DLL4 at approximately 90 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
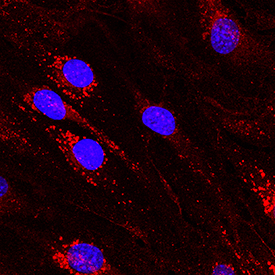 View Larger
View Larger
DLL4 in bEnd.3 Mouse Cell Line. DLL4 was detected in immersion fixed bEnd.3 mouse endothelioma cell line using Goat Anti-Mouse DLL4 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1389) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
DLL4 in Mouse Embryo. DLL4 was detected in immersion fixed paraffin-embedded sections of mouse embryo (13 d.p.c.) using Goat Anti-Mouse DLL4 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1389) at 5 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to developing vasculature. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: DLL4
Delta-Like protein 4 (DLL4) is a type I membrane protein belonging to the Delta/Serrate/Lag2 (DSL) family of Notch ligands (1). Notch signaling is an evolutionarily conserved pathway that controls cell fate and is required in multiple developmental processes including vascular development, hematopoiesis, somatogenesis, myogenesis, and neurogenesis (2-4). Dysregulation in the Notch pathway is associated with various human diseases. In mammals, four Notch homologs (Notch 1 to 4) and five ligands (DLL 1, 3 and 4, Jagged 1 and 2) have been identified. Notch ligands are transmembrane proteins with a DSL motif necessary for Notch binding, tandem EGF repeats, a transmembrane region and a short intracellular domain (ICD). Notch ligands are categorized into two subfamilies based on the presence of an extracellular cysteine-rich domain and insertions that interrupt some EGF repeats in the Jagged but not the Delta ligand family. Interactions of Notch receptors with their ligands results in reciprocal Regulated Intramembrane Proteolysis (RIP) (4). RIP is a mechanism for transmembrane signal transduction that involves the sequential processing by A Disintegrin Metalloprotease (ADAM) and then by Presenilin/ gamma -Ssecretase, resulting in shedding of the extracellular domains and the generation of the soluble ICD signaling fragments, respectively. The Notch ICD translocates to the nucleus and interacts with transcriptional coactivators, resulting in the transcription of target genes. The ICDs of the Notch ligands have also been shown to translocate to the nucleus where they may have a signaling function (5, 6). DLL4 is expressed highly and selectively within the arterial endothelium and has been shown to function as a ligand for Notch 1 and Notch 4. Human and mouse DLL4 share 86% amino acid sequence identity (1).
- Shutter, J.R. et al. (2000) Genes Dev. 14:1313.
- Iso, Tatsuya et al. (2002) Arterioscler. Thromb. Vasc. Biol. 23:543.
- Walker, L. et al. (2001) Stem Cells 19:543.
- Baron, M. (2002) Semin. Cell Dev. Biol. 14:113.
- Ikeuchi, T. and S.S. Sisodia (2003) J. Biol. Chem. 278:7751.
- Bland, C.E. et al. (2003) J. Biol. Chem. 278:13607.
Product Datasheets
Citations for Mouse DLL4 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
32
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
PI(4,5)P2-dependent regulation of endothelial tip cell specification contributes to angiogenesis
Authors: EM Davies, R Gurung, KQ Le, KTT Roan, RP Harvey, GM Mitchell, Q Schwarz, CA Mitchell
Science Advances, 2023-03-31;9(13):eadd6911.
Species: Mouse
Sample Types: Embryo
Applications: IHC -
Bacillus subtilis programs the differentiation of intestinal secretory lineages to inhibit Salmonella infection
Authors: Q Hou, J Jia, J Lin, L Zhu, S Xie, Q Yu, Y Li
Cell Reports, 2022-09-27;40(13):111416.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Gatekeeping role of Nf2/Merlin in vascular tip EC induction through suppression of VEGFR2 internalization
Authors: JH Bae, MJ Yang, SH Jeong, J Kim, SP Hong, JW Kim, YH Kim, GY Koh
Science Advances, 2022-06-10;8(23):eabn2611.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Induction of osteogenesis by bone-targeted Notch activation
Authors: C Xu, VV Dinh, K Kruse, HW Jeong, EC Watson, S Adams, F Berkenfeld, M Stehling, SJ Rasouli, R Fan, R Chen, I Bedzhov, Q Chen, K Kato, ME Pitulescu, RH Adams
Elife, 2022-02-04;11(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
The onset of circulation triggers a metabolic switch required for endothelial to hematopoietic transition
Authors: E Azzoni, V Frontera, G Anselmi, C Rode, C James, EM Deltcheva, AS Demian, J Brown, C Barone, A Patelli, JR Harman, M Nicholls, SJ Conway, E Morrissey, SEW Jacobsen, DB Sparrow, AL Harris, T Enver, MFTR de Bruijn
Cell Reports, 2021-12-14;37(11):110103.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models
Authors: DH Yeom, YS Lee, I Ryu, S Lee, B Sung, HB Lee, D Kim, JH Ahn, E Ha, YS Choi, SH Lee, WK You
International Journal of Molecular Sciences, 2020-12-29;22(1):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Heritable modifiers of the tumor microenvironment influence nanoparticle uptake, distribution and response to photothermal therapy
Authors: G Sharma, JM Jagtap, AK Parchur, VR Gogineni, S Ran, C Bergom, SB White, MJ Flister, A Joshi
Theranostics, 2020-04-06;10(12):5368-5383.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
BMP9 signaling promotes the normalization of tumor blood vessels
Authors: C Viallard, C Audiger, N Popovic, N Akla, K Lanthier, I Legault-Na, H Melichar, S Costantino, S Lesage, B Larrivée
Oncogene, 2020-02-10;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Endothelial ZEB1 promotes angiogenesis-dependent bone formation and reverses osteoporosis
Authors: R Fu, WC Lv, Y Xu, MY Gong, XJ Chen, N Jiang, Y Xu, QQ Yao, L Di, T Lu, LM Wang, R Mo, ZQ Wu
Nat Commun, 2020-01-23;11(1):460.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4
Authors: D Yasuda, D Kobayashi, N Akahoshi, T Ohto-Nakan, K Yoshioka, Y Takuwa, S Mizuno, S Takahashi, S Ishii
J. Clin. Invest., 2019-07-23;130(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Regulatory pathways governing murine coronary vessel formation are dysregulated in the injured adult heart
Authors: S Payne, M Gunadasa-R, A Neal, AN Redpath, J Patel, KM Chouliaras, I Ratnayaka, N Smart, S De Val
Nat Commun, 2019-07-22;10(1):3276.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Fibrotic liver microenvironment promotes Dll4 and SDF-1-dependent T-cell lineage development
Authors: Z Gong, B Shang, Y Chu, X Chen, Q Li, K Liu, Y Chen, Y Huang, Y Han, Q Shang, Z Zheng, L Song, Y Li, R Liu, C Xu, X Zhang, B Liu, L Wang, C Shao, Y Wang, Y Shi
Cell Death Dis, 2019-06-05;10(6):440.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC -
iSuRe-Cre is a genetic tool to reliably induce and report Cre-dependent genetic modifications
Authors: M Fernández-, V Casquero-G, W Luo, F Francesca, S Ferreira R, S Del Olmo-C, R Benedito
Nat Commun, 2019-05-22;10(1):2262.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-F -
Venous identity requires BMP signalling through ALK3
Authors: A Neal, S Nornes, S Payne, MD Wallace, M Fritzsche, P Louphrasit, RN Wilkinson, KM Chouliaras, K Liu, K Plant, R Sholapurka, I Ratnayaka, W Herzog, G Bond, T Chico, G Bou-Ghario, S De Val
Nat Commun, 2019-01-28;10(1):453.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
MPDZ promotes DLL4-induced Notch signaling during angiogenesis
Authors: F Tetzlaff, MG Adam, A Feldner, I Moll, A Menuchin, J Rodriguez-, D Sprinzak, A Fischer
Elife, 2018-04-05;7(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of Notch signalling and vascular stability
Authors: AV Shah, GM Birdsey, C Peghaire, ME Pitulescu, NP Dufton, Y Yang, I Weinberg, L Osuna Alma, L Payne, JC Mason, H Gerhardt, RH Adams, AM Randi
Nat Commun, 2017-07-11;8(0):16002.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC-Fr, Western Blot -
O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals
Authors: Sawaguchi S, Varshney S, Ogawa M et al.
eLife
-
Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation
Authors: RB Davis, DO Kechele, ES Blakeney, JB Pawlak, KM Caron
JCI Insight, 2017-03-23;2(6):e92465.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Data showing proliferation and differentiation of intestinal epithelial cells under targeted depletion of Notch ligands in mouse intestine.
Authors: Toru Nakata, Hiromichi Shimizu, Sayaka Nagata, Go Ito, Satoru Fujii, Kohei Suzuki, Ami Kawamoto, Fumiaki Ishibashi, Reiko Kuno, Sho Anzai, Tatsuro Murano, Tomohiro Mizutani, Shigeru Oshima, Kiichiro Tsuchiya, Tetsuya Nakamura, Katsuto Hozumi, Mamoru Watanabe, Ryuichi Okamoto
Data in Brief, 2016-12-29;0(0):2352-3409.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blood flow controls bone vascular function and osteogenesis
Nat Commun, 2016-12-06;7(0):13601.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Notch Signaling Regulates the Homeostasis of Tissue-Restricted Innate-like T Cells
Authors: Vijaykumar Chennupati
J Immunol, 2016-06-20;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Authors: Zhou Y, Williams J, Smallwood P, Nathans J
PLoS ONE, 2015-12-02;10(12):e0143650.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Interleukin-6 Stimulates Defective Angiogenesis.
Authors: Gopinathan G, Milagre C, Pearce O, Reynolds L, Hodivala-Dilke K, Leinster D, Zhong H, Hollingsworth R, Thompson R, Whiteford J, Balkwill F
Cancer Res, 2015-06-16;75(15):3098-107.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization.
Authors: Rama N, Dubrac A, Mathivet T, Ni Charthaigh R, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L, Eichmann A, Chedotal A
Nat Med, 2015-04-20;21(5):483-91.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Selective neuronal lineages derived from Dll4-expressing progenitors/precursors in the retina and spinal cord.
Authors: Zou M, Luo H, Xiang M
Dev Dyn, 2014-09-16;244(1):86-97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neuroligin 1 induces blood vessel maturation by cooperating with the alpha6 integrin.
Authors: Samarelli A, Riccitelli E, Bizzozero L, Silveira T, Seano G, Pergolizzi M, Vitagliano G, Cascone I, Carpentier G, Bottos A, Primo L, Bussolino F, Arese M
J Biol Chem, 2014-05-23;289(28):19466-76.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
Authors: Ramasamy S, Kusumbe A, Wang L, Adams R
Nature, 2014-03-12;507(7492):376-80.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Fbxw7 controls angiogenesis by regulating endothelial notch activity.
Authors: Izumi N, Helker C, Ehling M, Behrens A, Herzog W, Adams RH
PLoS ONE, 2012-07-27;7(7):e41116.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.
Authors: Benedito R, Rocha S, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams R
Nature, 2012-03-18;484(7392):110-4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system.
Authors: Stamataki D, Holder M, Hodgetts C
PLoS ONE, 2011-09-07;6(9):e24484.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Therapeutic efficacy of a DNA vaccine targeting the endothelial tip cell antigen delta-like ligand 4 in mammary carcinoma.
Authors: Haller BK, Brave A, Wallgard E, Roswall P, Sunkari VG, Mattson U, Hallengard D, Catrina SB, Hellstrom M, Pietras K
Oncogene, 2010-05-24;29(30):4276-86.
Species: Human, Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC-Fr, Western Blot -
Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation.
Authors: Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K
Nature, 2008-06-25;454(7204):656-60.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse DLL4 Antibody
There are currently no reviews for this product. Be the first to review Mouse DLL4 Antibody and earn rewards!
Have you used Mouse DLL4 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image


