Mouse/Rat IL-22 Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 35 | 35 | 35 |
| Mean (pg/mL) | 45.9 | 129 | 628 | 43.9 | 122 | 596 |
| Standard Deviation | 1.9 | 7.5 | 22.1 | 3.4 | 6.7 | 25.2 |
| CV% | 4.2 | 5.8 | 3.5 | 7.7 | 5.5 | 4.2 |
Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 34 | 34 | 34 |
| Mean (pg/mL) | 44.4 | 117 | 692 | 45.4 | 128 | 662 |
| Standard Deviation | 2 | 7 | 25.7 | 4.8 | 7.5 | 35.5 |
| CV% | 4.4 | 6 | 3.7 | 10.5 | 5.9 | 5.4 |
Recovery
The recovery of IL-22 spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Mouse Cell Culture Supernates (n=4) | 98 | 93-103 |
| Mouse EDTA Plasma (n=4) | 95 | 86-106 |
| Mouse Heparin Plasma (n=4) | 94 | 83-102 |
| Mouse Serum (n=4) | 91 | 84-99 |
| Rat Cell Culture Supernates (n=4) | 98 | 91-102 |
| Rat EDTA Plasma (n=4) | 97 | 91-111 |
| Rat Heparin Plasma (n=4) | 93 | 88-101 |
| Rat Serum (n=4) | 96 | 90-107 |
Linearity
Scientific Data
Preparation and Storage
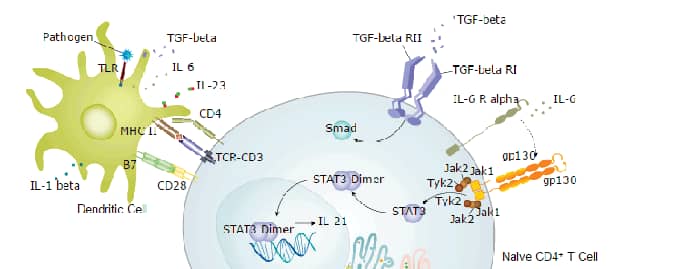
Background: IL-22
IL-22 (Interleukin-22) is a cytokine that induces the production of reactive oxygen species, IL-6, IL-10, and TNF-alpha as well as neutrophil infiltration during inflammation. It also supports the integrity of epithelial barriers and induces epithelial cell proliferation during wound healing. IL-22 signals through a receptor complex consisting of IL-22 R and IL-10 R beta. IL-10 R beta is a shared component of the receptor complexes for IL-10, IL-26, IL-28, and IL-29. IL-22 additionally binds to IL-22BP which blocks the interaction of IL-22 with IL-22 R.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 4 times for a total of 5 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours on the shaker.
- Aspirate and wash 5 times.
- Add 120 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 120 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Mouse/Rat IL-22 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
39
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A Dietary Oxysterol, 7-Ketocholesterol, Exacerbates Imiquimod-Induced Psoriasis-like Dermatitis in Steatohepatitic Mice
Authors: A Saga, M Koseki, K Kanno, J Chang, T Higo, D Okuzaki, T Okada, H Inui, M Asaji, K Tanaka, T Omatsu, S Nishihara, Y Zhu, K Ito, H Hattori, I Ichi, Y Kamada, M Ono, T Saibara, T Ohama, S Hikoso, M Nishida, S Yamashita, Y Sakata
International Journal of Molecular Sciences, 2022-12-13;23(24):.
Species: Mouse
Sample Types: Serum
-
Identification and characterization of innate lymphoid cells generated from pluripotent stem cells
Authors: J Xiong, Y Zhao, Y Lin, L Chen, Q Weng, C Shi, X Liu, Y Geng, L Liu, J Wang, M Zhang
Cell Reports, 2022-11-01;41(5):111569.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bone marrow transplantation induces changes in the gut microbiota that chronically increase the cytokine response pattern of splenocytes
Authors: S Katiraei, JA van Diepen, LP Tavares, LR Hoving, A Pronk, I Verschuere, PCN Rensen, JJ Zwaginga, S Kostidis, M Giera, M Teixera, KW van Dijk, MG Netea, JFP Berbée, V van Harmel
Scientific Reports, 2022-04-27;12(1):6883.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pharmacological Inhibition of Glutaminase 1 Attenuates Alkali-Induced Corneal Neovascularization by Modulating Macrophages
Authors: Y Feng, X Yang, J Huang, M Shen, L Wang, X Chen, Y Yuan, C Dong, X Ma, F Yuan
Oxidative Medicine and Cellular Longevity, 2022-03-19;2022(0):1106313.
Species: Mouse
Sample Types: Protein
-
Interleukin-22 mitigates acute respiratory distress syndrome (ARDS)
Authors: S Taghavi, O Jackson-We, S Abdullah, A Wanek, R Drury, J Packer, A Cotton-Bet, J Duchesne, D Pociask, J Kolls
PLoS ONE, 2021-10-01;16(10):e0254985.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neutralization of IL-33 modifies the type 2 and type 3 inflammatory signature of viral induced asthma exacerbation
Authors: KJ Warren, JA Poole, JM Sweeter, JM DeVasure, JD Dickinson, RS Peebles, TA Wyatt
Respiratory Research, 2021-07-15;22(1):206.
Species: Mouse
Sample Types: BALF
-
WLS/wntless is essential in controlling dendritic cell homeostasis via a WNT signaling-independent mechanism
Authors: LT Wang, MH Lin, KY Liu, SS Chiou, SN Wang, CY Chai, LW Tseng, HC Chiou, HC Wang, KK Yokoyama, SH Hsu, SK Huang
Autophagy, 2021-04-14;0(0):1-16.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Optic nerve regeneration screen identifies multiple genes restricting adult neural repair
Authors: JA Lindborg, NM Tran, DM Chenette, K DeLuca, Y Foli, R Kannan, Y Sekine, X Wang, M Wollan, IJ Kim, JR Sanes, SM Strittmatt
Cell Reports, 2021-03-02;34(9):108777.
Species: Mouse
Sample Types: Tissue Homogenates
-
A New Mouse Model of Chronic Myocarditis Induced by Recombinant Bacille Calmette-Gu�rin Expressing a T-Cell Epitope of Cardiac Myosin Heavy Chain-&alpha
Authors: K Tajiri, K Imanaka-Yo, Y Tsujimura, K Matsuo, M Hiroe, K Aonuma, M Ieda, Y Yasutomi
International Journal of Molecular Sciences, 2021-01-14;22(2):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 Induce the Expression of the REG3 Family in the Small Intestine of Mice via the Stimulation of Dendritic Cells and Type 3 Innate Lymphoid Cells
Authors: K Kobayashi, Y Honme, T Sashihara
Nutrients, 2019-12-07;11(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The cyclooxygenase-1/mPGES-1/endothelial prostaglandin EP4 receptor pathway constrains myocardial ischemia-reperfusion injury
Authors: L Zhu, C Xu, X Huo, H Hao, Q Wan, H Chen, X Zhang, RM Breyer, Y Huang, X Cao, DP Liu, GA FitzGerald, M Wang
Nat Commun, 2019-04-23;10(1):1888.
Species: Mouse
Sample Types: Plasma
-
PYY plays a key role in the resolution of diabetes following bariatric surgery in humans
Authors: C Guida, SD Stephen, M Watson, N Dempster, P Larraufie, T Marjot, T Cargill, L Rickers, M Pavlides, J Tomlinson, JFL Cobbold, CM Zhao, D Chen, F Gribble, F Reimann, R Gillies, B Sgromo, P Rorsman, JD Ryan, RD Ramracheya
EBioMedicine, 2019-01-11;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Multi-Walled Carbon Nanotubes Augment Allergic Airway Eosinophilic Inflammation by Promoting Cysteinyl Leukotriene Production
Authors: S Carvalho, M Ferrini, L Herritt, A Holian, Z Jaffar, K Roberts
Front Pharmacol, 2018-06-05;9(0):585.
Species: Mouse
Sample Types: BALF
-
Fungal immunomodulatory protein-fve could modulate airway remodel through by affect IL17 cytokine
Authors: YT Lee, CT Wu, HL Sun, JL Ko, KH Lue
J Microbiol Immunol Infect, 2017-06-29;0(0):.
Species: Mouse
Sample Types: BALF
-
Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer's patches
Authors: SH Kim, BH Cho, H Kiyono, YS Jang
Sci Rep, 2017-06-21;7(1):3980.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ablation of IL-17 expression moderates experimental autoimmune myasthenia gravis disease severity
Authors: G Aguilo-Sea, Y Xie, J Sheehan, LL Kusner, HJ Kaminski
Cytokine, 2017-06-06;96(0):279-285.
Species: Mouse
Sample Types: Serum
-
STAT1 Represses Cytokine-Producing Group 2 and Group 3 Innate Lymphoid Cells during Viral Infection
Authors: MT Stier, K Goleniewsk, JY Cephus, DC Newcomb, TP Sherrill, KL Boyd, MH Bloodworth, ML Moore, K Chen, JK Kolls, RS Peebles
J. Immunol., 2017-06-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Biological effects of bone marrow mesenchymal stem cells on hepatitis B virus in�vitro
Authors: WP Zheng, BY Zhang, ZY Shen, ML Yin, Y Cao, HL Song
Mol Med Rep, 2017-03-15;15(5):2551-2559.
Species: Rat
Sample Types: Cell Culture Supernates
-
IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance.
Authors: Basu R, Whitley S, Bhaumik S, Zindl C, Schoeb T, Benveniste E, Pear W, Hatton R, Weaver C
Nat Immunol, 2015-02-02;16(3):286-95.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Prostaglandin E2 negatively regulates the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis.
Authors: Saha A, Biswas A, Srivastav S, Mukherjee M, Das P, Ukil A
J Immunol, 2014-07-21;193(5):2330-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner.
Authors: Dulek D, Newcomb D, Goleniewska K, Cephus J, Zhou W, Reiss S, Toki S, Ye F, Zaynagetdinov R, Sherrill T, Blackwell T, Moore M, Boyd K, Kolls J, Peebles R
Infect Immun, 2014-06-23;82(9):3723-39.
Species: Mouse
Sample Types: Tissue Homogenates
-
Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis.
Authors: Qin S, Wen J, Bai X, Chen T, Zheng R, Zhou G, Ma J, Feng J, Zhong B, Li Y
Mol Med Rep, 2014-04-09;9(6):2097-104.
Species: Mouse
Sample Types: Serum
-
The Acute Neutrophil Response Mediated by S100 Alarmins during Vaginal Candida Infections Is Independent of the Th17-Pathway.
Authors: Yano, Junko, Kolls, Jay K, Happel, Kyle I, Wormley, Floyd, Wozniak, Karen L, Fidel, Paul L J
PLoS ONE, 2012-09-25;7(9):e46311.
Species: Mouse
Sample Types: Vaginal Lavage Fluid
-
IL-22 signaling contributes to West Nile encephalitis pathogenesis.
Authors: Wang, Penghua, Bai, Fengwei, Zenewicz, Lauren A, Dai, Jianfeng, Gate, David, Cheng, Gong, Yang, Long, Qian, Feng, Yuan, Xiaoling, Montgomery, Ruth R, Flavell, Richard, Town, Terrence, Fikrig, Erol
PLoS ONE, 2012-08-28;7(8):e44153.
Species: Mouse
Sample Types: Plasma
-
The Receptor Slamf1 on the Surface of Myeloid Lineage Cells Controls Susceptibility to Infection by Trypanosoma cruzi.
Authors: Calderon J, Maganto-Garcia E, Punzon C, Carrion J, Terhorst C, Fresno M
PLoS Pathog., 2012-07-12;8(7):e1002799.
Species: Mouse
Sample Types: Serum
-
Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus.
Authors: Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C
Infect. Immun., 2011-10-28;80(1):410-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy.
Authors: Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L
J. Exp. Med., 2011-03-07;208(3):491-503.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-23-Mediated Epidermal Hyperplasia Is Dependent on IL-6.
Authors: Lindroos J, Svensson L, Norsgaard H
J. Invest. Dermatol., 2011-02-03;131(5):1110-8.
Species: Mouse
Sample Types: Tissue Homogenates
-
Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice.
Authors: Kudva A, Scheller EV, Robinson KM
J. Immunol., 2010-12-22;186(3):1666-74.
Species: Mouse
Sample Types: BALF
-
A novel role for IL-22R1 as a driver of inflammation.
Authors: Savan R, McFarland AP, Reynolds DA
Blood, 2010-10-22;117(2):575-84.
Species: Mouse
Sample Types: Serum
-
IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans.
Authors: Kagami S, Rizzo HL, Kurtz SE
J. Immunol., 2010-10-04;185(9):5453-62.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dexamethasone suppresses interleukin-22 associated with bacterial infection in vitro and in vivo.
Authors: Ziesche E, Scheiermann P, Bachmann M, Sadik CD, Hofstetter C, Zwissler B, Pfeilschifter J, Muhl H
Clin. Exp. Immunol., 2009-09-01;157(3):370-6.
Species: Mouse
Sample Types: Plasma
-
Phosphodiesterase 7A inhibitor ASB16165 suppresses proliferation and cytokine production of NKT cells.
Authors: Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Inoue H, Murafuji H, Hayashi Y, Miura K, Nakatsuka T, Nagahira K, Chamoto K, Fukuda Y, Nishimura T
Cell. Immunol., 2009-05-27;258(2):147-51.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-22-dependent attenuation of T cell-dependent (ConA) hepatitis in herpes virus entry mediator deficiency.
Authors: Wahl C, Wegenka UM, Leithauser F, Schirmbeck R, Reimann J
J. Immunol., 2009-04-15;182(8):4521-8.
Species: Mouse
Sample Types: Serum
-
Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis.
Authors: Siegemund S, Schutze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, Sabat R, Alber G
Int. Immunol., 2009-03-18;21(5):555-65.
Species: Mouse
Sample Types: Serum
-
TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice.
Authors: McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK
J. Immunol., 2008-09-15;181(6):4089-97.
Species: Mouse
Sample Types: BALF
-
IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia.
Authors: Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK
Nat. Med., 2008-02-10;14(3):275-81.
Species: Mouse
Sample Types: Tissue Homogenates
-
TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology.
Authors: McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ
Nat. Immunol., 2007-11-11;8(12):1390-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4.
Authors: Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M
Nat. Immunol., 2007-08-05;8(9):958-66.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse/Rat IL-22 Quantikine ELISA Kit
There are currently no reviews for this product. Be the first to review Mouse/Rat IL-22 Quantikine ELISA Kit and earn rewards!
Have you used Mouse/Rat IL-22 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image