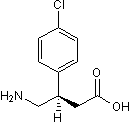
(R)-Baclofen
Chemical Name: (R)-4-Amino-3-(4-chlorophenyl)butanoic acid
Purity: ≥99%
Biological Activity
More active enantiomer of (RS)-Baclofen, a selective GABAB agonist.Racemate also available.
Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Additional Information
Background References
-
Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations.
Falch et al.
J.Neurochem., 1986;47:898 -
Effects of phaclofen and the enantiomers of bac. on cardiovascular responses to intrathecal administration of L- and D-baclofen in the rat.
Hong et al.
Eur.J.Pharmacol., 1991;196:267
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for (R)-Baclofen
The citations listed below are publications that use Tocris products. Selected citations for (R)-Baclofen include:
14 Citations: Showing 1 - 10
-
Mitochondrial Regulation of the Hippocampal Firing Rate Set Point and Seizure Susceptibility.
Authors: Styr Et al.
Neuron 2019;102:1009
-
Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence.
Authors: Chai Et al.
Neuron 2017;95:531
-
Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals.
Authors: Bragina Et al.
Front Cell Neurosci 2015;9:345
-
Neuronal γ-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA.
Authors: Wang Et al.
Mol Cell Neurosci 2015;9:188
-
GABAB receptor upregulates fragile X mental retardation protein expression in neurons.
Authors: Zhang Et al.
Front Cell Neurosci 2015;5:10468
-
Development and function of human cerebral cortex neural networks from pluripotent stem cells in vitro.
Authors: Kirwan Et al.
J Pharmacol Exp Ther 2015;142:3178
-
Regulation of action potential waveforms by axonal GABAA receptors in cortical pyramidal neurons.
Authors: Xia Et al.
PLoS One 2014;9:e100968
-
Epileptiform activity in the CA1 region of the hippocampus becomes refractory to attenuation by cannabinoids in part because of endogenous γ-aminobutyric acid type B receptor activity.
Authors: Messer and Levine
J Neurosci Res 2012;90:1454
-
Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability.
Authors: Klug Et al.
PLoS One 2012;7:e45323
-
GABAB receptor-positive modulators: brain region-dependent effects.
Authors: Hensler Et al.
J Pharmacol Exp Ther 2012;340:19
-
Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome.
Authors: Pacey Et al.
Sci Rep 2011;338:897
-
GABAB receptor constituents revealed by tandem affinity purification from transgenic mice.
Authors: Bartoi Et al.
J Biol Chem 2010;285:20625
-
A dynamic role for GABA receptors on the firing pattern of midbrain DArgic neurons.
Authors: Lobb Et al.
J Neurophysiol 2010;104:403
-
Peripheral GABAB agonists stimulate gastric acid secretion in mice.
Authors: Piqueras and Martínez
Br J Pharmacol 2004;142:1038
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for (R)-Baclofen
There are currently no reviews for this product. Be the first to review (R)-Baclofen and earn rewards!
Have you used (R)-Baclofen?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image