Recombinant Human Flt-3 Ligand/FLT3L Protein
NEW Next Generation Products! Please try our NEW version of Human Recombinant Flt-3 Ligand (308-FKHB). Combining R&D Systems quality with scalability that allows for lower price points and a solid supply chain.
Recombinant Human Flt-3 Ligand/FLT3L Protein Summary
Product Specifications
Thr27-Pro185
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
308-FK
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
308-FK/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA. |
| Reconstitution | Reconstitute 5 µg vials at 50 µg/mL in sterile PBS. Reconstitute 25 µg or larger vials at 100 µg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
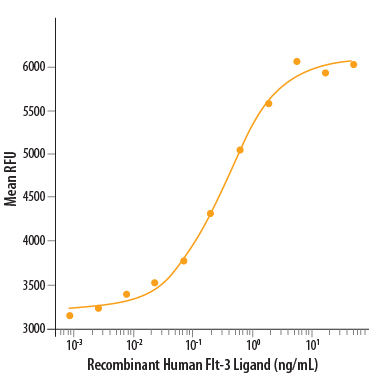
Recombinant Human Flt-3 Ligand/FLT3L (Catalog # 308-FK) stimulates cell proliferation in the BaF3 mouse pro-B cell line transfected with mouse Flt-3. The ED50 for this effect is 0.2-1 ng/mL.
 View Larger
View Larger
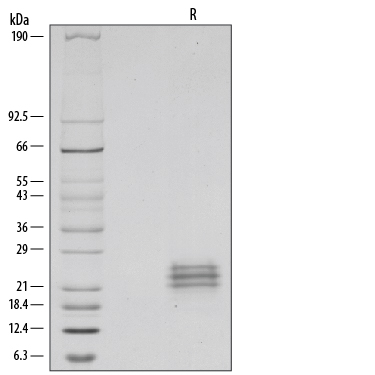
1 µg/lane of Recombinant Human Flt-3 Ligand/FLT3L was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing major bands at 22‑26 kDa. The multiple bands are due to variable glycosylation of the protein.
 View Larger
View Larger
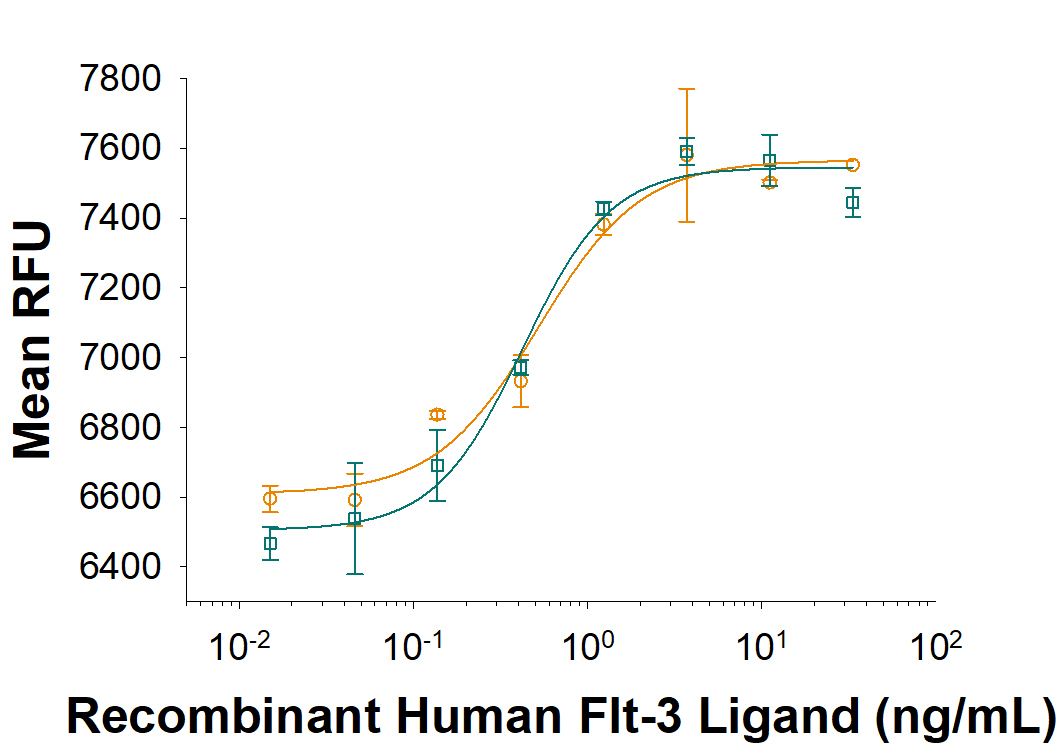
Equivalent bioactivity of Sf21-derived RUO (Catalog # 308-FK) and HEK293-derived RUO (308-FKHB) Recombinant Human Flt-3 Ligand as measured in a cell proliferation assay using the cell proliferation of BaF3 mouse pro-B cell line transfected with mouse Flt-3. (orange,green, respectively).
Reconstitution Calculator
Background: Flt-3 Ligand/FLT3L
Flt‑3 Ligand, also known as FLT3L, is an alpha-helical cytokine that promotes the differentiation of multiple hematopoietic cell lineages (1-3). Mature human Flt‑3 Ligand consists of a 158 amino acid (aa) extracellular domain (ECD) with a cytokine-like domain and a juxtamembrane tether region, a 21 aa transmembrane segment, and a 30 aa cytoplasmic tail (4-7). Within the ECD, human Flt‑3 Ligand shares 71% and 65% aa sequence identity with mouse and rat Flt‑3 Ligand, respectively (4-6). The human and mouse Flt‑3 Ligand proteins show cross-species activity. Flt-3 Ligand is also structurally related to M-CSF and SCF. Flt-3 Ligand is widely expressed in various human and mouse tissues. It is expressed as a noncovalently-linked dimer by T cells and bone marrow and thymic fibroblasts (1, 8). Each 36 kDa chain of the Flt-3 Ligand dimer carries approximately 12 kDa of N- and O-linked carbohydrates (8). Alternate splicing and proteolytic cleavage of the transmembrane form of the Flt-3 Ligand protein can generate a soluble 30 kDa fragment that includes the cytokine-like domain (4, 8). Alternate splicing of human Flt‑3 Ligand also generates membrane-associated isoforms that contain either a truncated cytoplasmic tail or an 85 aa substitution following the cytokine-like domain in the ECD of the Flt-3 Ligand protein (4, 5, 8). Both transmembrane and soluble forms of Flt‑3 Ligand signal through the tyrosine kinase receptor Flt-3/Flk-2 (3, 4, 6, 7). Flt‑3 Ligand induces the expansion of monocytes and immature dendritic cells as well as early B cell lineage differentiation (2, 9). Additionally, Flt-3 Ligand synergizes with IL-3, GM-CSF, and SCF to promote the mobilization and myeloid differentiation of hematopoietic stem cells (4-6). Flt-3 Ligand also cooperates with IL-2, IL-6, IL-7, and IL-15 to induce NK cell development and with IL-3, IL-7, and IL-11 to induce terminal B cell maturation (1, 10). Animal studies show that Flt‑3 Ligand reduces the severity of experimentally induced allergic inflammation (11).
- Wodnar-Filipowicz, A. (2003) News Physiol. Sci. 18:247.
- Dong, J. et al. (2002) Cancer Biol. Ther. 1:486.
- Gilliland, D.G. and J.D. Griffin (2002) Blood 100:1532.
- Hannum, C. et al. (1994) Nature 368:643.
- Lyman, S.D. et al. (1994) Blood 83:2795.
- Lyman, S.D. et al. (1993) Cell 75:1157.
- Savvides, S.N. et al. (2000) Nat. Struct. Biol. 7:486.
- McClanahan, T. et al. (1996) Blood 88:3371.
- Diener, K.R. et al. (2008) Exp. Hematol. 36:51.
- Farag, S.S. and M.A. Caligiuri (2006) Blood Rev. 20:123.
- Edwan, J.H. et al. (2004) J. Immunol. 172:5016.
Citations for Recombinant Human Flt-3 Ligand/FLT3L Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
62
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Strength of CAR signaling determines T�cell versus ILC differentiation from pluripotent stem cells
Authors: S Li, CS Wang, A Montel-Hag, HC Chen, S Lopez, O Zhou, K Dai, S Tsai, W Satyadi, C Botero, C Wong, D Casero, GM Crooks, CS Seet
Cell Reports, 2023-03-11;42(3):112241.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Improved Sendai viral system for reprogramming to naive pluripotency.
Authors: Kunitomi A, Hirohata R, Arreola V, Osawa M, Kato T, Nomura M, Kawaguchi J, Hara H, Kusano K, Takashima Y, Takahashi K, Fukuda K, Takasu N, Yamanaka S
Cell Rep Methods, 2022-10-17;2(11):100317.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
DLL4 and VCAM1 enhance the emergence of T cell-competent hematopoietic progenitors from human pluripotent stem cells
Authors: YS Michaels, JM Edgar, MC Major, EL Castle, C Zimmerman, T Yin, A Hagner, C Lau, HH Hsu, MI Ibañez-Rio, LJ Durland, DJHF Knapp, PW Zandstra
Science Advances, 2022-08-24;8(34):eabn5522.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
EZH1 repression generates mature iPSC-derived CAR T�cells with enhanced antitumor activity
Authors: R Jing, I Scarfo, MA Najia, E Lummertz d, A Han, M Sanborn, T Bingham, C Kubaczka, DK Jha, M Falchetti, TM Schlaeger, TE North, MV Maus, GQ Daley
Cell Stem Cell, 2022-08-04;29(8):1181-1196.e6.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Clinical evolution, genetic landscape and trajectories of clonal hematopoiesis in SAMD9/SAMD9L syndromes
Authors: SS Sahoo, VB Pastor, C Goodings, RK Voss, EJ Kozyra, A Szvetnik, P Noellke, M Dworzak, J Starý, F Locatelli, R Masetti, M Schmugge, B De Moerloo, A Catala, K Kállay, D Turkiewicz, H Hasle, J Buechner, K Jahnukaine, M Ussowicz, S Polychrono, OP Smith, O Fabri, S Barzilai, V de Haas, I Baumann, S Schwarz-Fu, European W, MR Niewisch, MG Sauer, B Burkhardt, P Lang, P Bader, R Beier, I Müller, MH Albert, R Meisel, A Schulz, G Cario, PK Panda, J Wehrle, S Hirabayash, M Derecka, R Durruthy-D, G Göhring, A Yoshimi-No, M Ku, D Lebrecht, M Erlacher, C Flotho, B Strahm, CM Niemeyer, MW Wlodarski
Nature Medicine, 2021-10-07;27(10):1806-1817.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Base editing of haematopoietic stem cells rescues sickle cell disease in mice
Authors: GA Newby, JS Yen, KJ Woodard, T Mayuranath, CR Lazzarotto, Y Li, H Sheppard-T, SN Porter, Y Yao, K Mayberry, KA Everette, Y Jang, CJ Podracky, E Thaman, C Lechauve, A Sharma, JM Henderson, MF Richter, KT Zhao, SM Miller, T Wang, LW Koblan, AP McCaffrey, JF Tisdale, TA Kalfa, SM Pruett-Mil, SQ Tsai, MJ Weiss, DR Liu
Nature, 2021-06-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human pluripotent stem cells identify molecular targets of trisomy 12 in chronic lymphocytic leukemia patients
Authors: JC Reid, D Golubeva, AL Boyd, CG Hollands, C Henly, L Orlando, A Leber, J Hébert, F Morabito, G Cutrona, L Agnelli, M Gentile, M Ferrarini, A Neri, B Leber, M Bhatia
Cell Reports, 2021-03-16;34(11):108845.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Sequential CRISPR gene editing in human iPSCs charts the clonal evolution of myeloid leukemia and identifies early disease targets
Authors: T Wang, AR Pine, AG Kotini, H Yuan, L Zamparo, DT Starczynow, C Leslie, EP Papapetrou
Cell Stem Cell, 2021-02-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Aberrant expression of NKL homeobox genes HMX2 and HMX3 interferes with cell differentiation in acute myeloid leukemia
Authors: S Nagel, C Pommerenke, C Meyer, RAF MacLeod, HG Drexler
PLoS ONE, 2020-10-13;15(10):e0240120.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Acute Myeloid Leukemia iPSCs Reveal a Role for RUNX1 in the Maintenance of Human Leukemia Stem Cells
Authors: J Wesely, AG Kotini, F Izzo, H Luo, H Yuan, J Sun, M Georgomano, A Zviran, AG Deslaurier, N Dusaj, SD Nimer, C Leslie, DA Landau, MG Kharas, EP Papapetrou
Cell Rep, 2020-06-02;31(9):107688.
Species: Human
Sample Types: Whole Cell
-
Low-Dose Busulfan Reduces Human CD34+ Cell Doses Required for Engraftment in c-kit Mutant Immunodeficient Mice
Authors: A Leonard, M Yapundich, T Nassehi, J Gamer, CM Drysdale, JJ Haro-Mora, S Demirci, MM Hsieh, N Uchida, JF Tisdale
Mol Ther Methods Clin Dev, 2019-11-11;15(0):430-437.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Genome-wide microhomologies enable precise template-free editing of biologically relevant deletion mutations
Authors: J Grajcarek, J Monlong, Y Nishinaka-, M Nakamura, M Nagai, S Matsuo, D Lougheed, H Sakurai, MK Saito, G Bourque, K Woltjen
Nat Commun, 2019-10-24;10(1):4856.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Development of a forward-oriented therapeutic lentiviral vector for hemoglobin disorders
Authors: N Uchida, MM Hsieh, L Raines, JJ Haro-Mora, S Demirci, AC Bonifacino, AE Krouse, ME Metzger, RE Donahue, JF Tisdale
Nat Commun, 2019-10-02;10(1):4479.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Diminished AHR signaling drives human acute myeloid leukemia stem cell maintenance
Authors: M Ly, S Rentas, A Vujovic, N Wong, S Moreira, J Xu, N Holzapfel, S Bhatia, D Tran, MD Minden, JS Draper, KJ Hope
Cancer Res., 2019-09-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Biosafety Studies of a Clinically Applicable Lentiviral Vector for the Gene Therapy of Artemis-SCID
Authors: S Charrier, C Lagresle-P, V Poletti, M Rothe, G Cédrone, B Gjata, F Mavilio, A Fischer, A Schambach, JP de Villart, M Cavazzana, S Hacein-Bey, A Galy
Mol Ther Methods Clin Dev, 2019-09-13;15(0):232-245.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Homologous Recombination-Based Genome Editing by Clade F AAVs Is Inefficient in the Absence of a Targeted DNA Break
Authors: GL Rogers, HY Chen, H Morales, PM Cannon
Mol. Ther., 2019-09-09;27(10):1726-1736.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interconversion between Tumorigenic and Differentiated States in Acute Myeloid Leukemia
Authors: MD McKenzie, M Ghisi, EP Oxley, S Ngo, L Cimmino, C Esnault, R Liu, JM Salmon, CC Bell, N Ahmed, M Erlichster, MT Witkowski, GJ Liu, M Chopin, A Dakic, E Simankowic, G Pomilio, T Vu, P Krsmanovic, S Su, L Tian, TM Baldwin, DA Zalcenstei, L DiRago, S Wang, D Metcalf, RW Johnstone, BA Croker, GI Lancaster, AJ Murphy, SH Naik, SL Nutt, V Pospisil, T Schroeder, M Wall, MA Dawson, AH Wei, H de Thé, ME Ritchie, J Zuber, RA Dickins
Cell Stem Cell, 2019-08-01;25(2):258-272.e9.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
CRISPR/Cas9 mediated ELANE knockout enables neutrophilic maturation of primary hematopoietic stem and progenitor cells and induced pluripotent stem cells of severe congenital neutropenia patients
Authors: M Nasri, M Ritter, P Mir, B Dannenmann, N Aghaallaei, D Amend, V Makaryan, Y Xu, B Fletcher, R Bernhard, I Steiert, K Hahnel, J Berger, I Koch, B Sailer, K Hipp, C Zeidler, M Klimiankou, B Bajoghli, DC Dale, K Welte, J Skokowa
Haematologica, 2019-06-27;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Hemogenic Endothelium Differentiation from Human Pluripotent Stem Cells in A Feeder- and Xeno-free Defined Condition.
Authors: Ohta R, Sugimura R, Niwa A, Saito M
J Vis Exp, 2019-06-16;0(148):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Therapeutic discovery for marrow failure with MDS predisposition using pluripotent stem cells
Authors: M Ruiz-Gutie, ÖV Bölükba??, G Alexe, AG Kotini, K Ballotti, CE Joyce, DW Russell, K Stegmaier, K Myers, CD Novina, EP Papapetrou, A Shimamura
JCI Insight, 2019-04-30;5(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Generation of a human induced pluripotent stem cell line, BRCi001-A, derived from a patient with mucopolysaccharidosis type I
Authors: M Suga, T Kondo, K Imamura, R Shibukawa, Y Okanishi, Y Sagara, K Tsukita, T Enami, M Furujo, K Saijo, Y Nakamura, M Osawa, MK Saito, S Yamanaka, H Inoue
Stem Cell Res, 2019-02-12;36(0):101406.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
High-Efficiency Lentiviral Transduction of Human CD34+ Cells in High-Density Culture with Poloxamer and Prostaglandin E2
Authors: N Uchida, T Nassehi, CM Drysdale, J Gamer, M Yapundich, S Demirci, JJ Haro-Mora, A Leonard, MM Hsieh, JF Tisdale
Mol Ther Methods Clin Dev, 2019-01-25;13(0):187-196.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
An integration-free iPSC line (MUSIi008-A) derived from a patient with severe hemolytic anemia carrying compound heterozygote mutations in KLF1 gene for disease modeling
Authors: P Potirat, M Wattanapan, V Viprakasit, P Kheolamai, S Issaragris
Stem Cell Res, 2018-12-14;34(0):101344.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Restricted cell cycle is essential for clonal evolution and therapeutic resistance of pre-leukemic stem cells
Authors: CS Tremblay, J Saw, SK Chiu, NC Wong, K Tsyganov, S Ghotb, AN Graham, F Yan, AA Guirguis, SE Sonderegge, N Lee, P Kalitsis, J Reynolds, SB Ting, DR Powell, SM Jane, DJ Curtis
Nat Commun, 2018-08-30;9(1):3535.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
STK3 is a therapeutic target for a subset of acute myeloid leukemias
Authors: A Camgoz, M Paszkowski, S Satpathy, M Wermke, MV Hamann, M von Bonin, C Choudhary, S Knapp, F Buchholz
Oncotarget, 2018-05-22;9(39):25458-25473.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human Cytomegalovirus Encodes a Novel FLT3 Receptor Ligand Necessary for Hematopoietic Cell Differentiation and Viral Reactivation
Authors: LB Crawford, JH Kim, D Collins-Mc, BJ Lee, I Landais, C Held, JA Nelson, AD Yurochko, P Caposio
MBio, 2018-04-24;9(2):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A CLK3-HMGA2 Alternative Splicing Axis Impacts Human Hematopoietic Stem Cell Molecular Identity throughout Development
Authors: M Cesana, MH Guo, D Cacchiarel, L Wahlster, J Barragan, S Doulatov, LT Vo, B Salvatori, C Trapnell, K Clement, P Cahan, KM Tsanov, PM Sousa, B Tazon-Vega, A Bolondi, FM Giorgi, A Califano, JL Rinn, A Meissner, JN Hirschhorn, GQ Daley
Cell Stem Cell, 2018-04-05;22(4):575-588.e7.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Molecular transitions in early progenitors during human cord blood hematopoiesis
Authors: S Zheng, E Papalexi, A Butler, W Stephenson, R Satija
Mol. Syst. Biol., 2018-03-15;14(3):e8041.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
BRD3/4 inhibition and FLT3-ligand deprivation target pathways that are essential for the survival of human MLL-AF9+ leukemic cells
Authors: M Carretta, AZ Brouwers-V, M Bosman, SJ Horton, JHA Martens, E Vellenga, JJ Schuringa
PLoS ONE, 2017-12-14;12(12):e0189102.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Polycomb protein RING1A limits hematopoietic differentiation in myelodysplastic syndromes
Authors: A Palau, AK Garz, J Diesch, A Zwick, R Malinverni, V Valero, K Lappin, R Casquero, A Lennartsso, J Zuber, T Navarro, KI Mills, KS Götze, M Buschbeck
Oncotarget, 2017-12-01;8(70):115002-115017.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Targeting of Immune Cells by Dual TLR2/7 Ligands Suppresses Features of Allergic Th2 Immune Responses in Mice
Authors: J Laiño, A Wangorsch, F Blanco, S Wolfheimer, M Krause, A Flaczyk, TM Möller, M Tsai, S Galli, S Vieths, M Toda, S Scheurer, S Schülke
J Immunol Res, 2017-10-24;2017(0):7983217.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Let-7 microRNA-dependent control of leukotriene signaling regulates the transition of hematopoietic niche in mice
Authors: X Jiang, JS Hawkins, J Lee, CO Lizama, FL Bos, JP Zape, P Ghatpande, Y Peng, J Louie, G Lagna, AC Zovein, A Hata
Nat Commun, 2017-07-25;8(1):128.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A novel approach for the identification of efficient combination therapies in primary human acute myeloid leukemia specimens
Authors: I Baccelli, J Krosl, G Boucher, I Boivin, VP Lavallée, J Hébert, S Lemieux, A Marinier, G Sauvageau
Blood Cancer J, 2017-02-17;7(2):e529.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Stage-Specific Human Induced Pluripotent Stem Cells Map the Progression of Myeloid Transformation to Transplantable Leukemia
Authors: AG Kotini, CJ Chang, A Chow, H Yuan, TC Ho, T Wang, S Vora, A Solovyov, C Husser, M Olszewska, J Teruya-Fel, D Perumal, VM Klimek, A Spyridonid, RK Rampal, L Silverman, EP Reddy, E Papaemmanu, S Parekh, BD Greenbaum, CS Leslie, MG Kharas, EP Papapetrou
Cell Stem Cell, 2017-02-16;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease
JCI Insight, 2016-12-08;1(20):e87446.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer.
Authors: Hermanson D, Bendzick L, Pribyl L, McCullar V, Vogel R, Miller J, Geller M, Kaufman D
Stem Cells, 2015-12-06;34(1):93-101.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Prospectively Isolated Human Bone Marrow Cell-Derived MSCs Support Primitive Human CD34-Negative Hematopoietic Stem Cells.
Authors: Matsuoka Y, Nakatsuka R, Sumide K, Kawamura H, Takahashi M, Fujioka T, Uemura Y, Asano H, Sasaki Y, Inoue M, Ogawa H, Takahashi T, Hino M, Sonoda Y
Stem Cells, 2015-05-01;33(5):1554-65.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Functional Characterization of D9, a Novel Deazaneplanocin A (DZNep) Analog, in Targeting Acute Myeloid Leukemia (AML).
Authors: Jiang X, Lim C, Li Z, Lee P, Yatim S, Guan P, Li J, Zhou J, Pan J, Chng W, Chai C, Yu Q
PLoS ONE, 2015-04-30;10(4):e0122983.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reversible lineage-specific priming of human embryonic stem cells can be exploited to optimize the yield of differentiated cells.
Authors: Lee J, Graham M, Collins T, Lee J, Hong S, McNicol A, Shapovalova Z, Bhatia M
Stem Cells, 2015-04-01;33(4):1142-52.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques.
Authors: Barese C, Felizardo T, Sellers S, Keyvanfar K, Di Stasi A, Metzger M, Krouse A, Donahue R, Spencer D, Dunbar C
Stem Cells, 2015-01-01;33(1):91-100.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
SCL, LMO1 and Notch1 reprogram thymocytes into self-renewing cells.
Authors: Gerby B, Tremblay C, Tremblay M, Rojas-Sutterlin S, Herblot S, Hebert J, Sauvageau G, Lemieux S, Lecuyer E, Veiga D, Hoang T
PLoS Genet, 2014-12-18;10(12):e1004768.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Small-molecule inhibitor of glycogen synthase kinase 3beta 6-Bromoindirubin-3-oxime inhibits hematopoietic regeneration in stem cell recipient mice.
Authors: Shen S, Xu N, Klamer G, Ko K, Khoo M, Ma D, Moore J, O'Brien T, Dolnikov A
Stem Cells Dev, 2014-12-09;24(6):724-36.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human induced pluripotent stem cell derived erythroblasts can undergo definitive erythropoiesis and co-express gamma and beta globins.
Authors: Yang C, French A, Goh P, Pagnamenta A, Mettananda S, Taylor J, Knight S, Nathwani A, Roberts D, Watt S, Carpenter L
Br J Haematol, 2014-05-16;166(3):435-48.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells.
Authors: Kamat V, Paluru P, Myint M, French D, Gadue P, Diamond S
Stem Cells, 2014-05-01;32(5):1337-46.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells.
Authors: Wu C, Li B, Lu R, Koelle S, Yang Y, Jares A, Krouse A, Metzger M, Liang F, Lore K, Wu C, Donahue R, Chen I, Weissman I, Dunbar C
Cell Stem Cell, 2014-04-03;14(4):486-99.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
Repression of BIM mediates survival signaling by MYC and AKT in high-risk T-cell acute lymphoblastic leukemia.
Authors: Reynolds, C, Roderick, J E, LaBelle, J L, Bird, G, Mathieu, R, Bodaar, K, Colon, D, Pyati, U, Stevenson, K E, Qi, J, Harris, M, Silverman, L B, Sallan, S E, Bradner, J E, Neuberg, D S, Look, A T, Walensky, L D, Kelliher, M A, Gutierrez, A
Leukemia, 2014-02-20;28(9):1819-27.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A lack of secretory leukocyte protease inhibitor (SLPI) causes defects in granulocytic differentiation.
Authors: Klimenkova O, Ellerbeck W, Klimiankou M, Unalan M, Kandabarau S, Gigina A, Hussein K, Zeidler C, Welte K, Skokowa J
Blood, 2013-12-18;123(8):1239-49.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa.
Authors: Tolar J, McGrath J, Xia L, Riddle M, Lees C, Eide C, Keene D, Liu L, Osborn M, Lund T, Blazar B, Wagner J
J Invest Dermatol, 2013-12-06;134(5):1246-54.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Characterization of CD56+ dendritic-like cells: a normal counterpart of blastic plasmacytoid dendritic cell neoplasm?
Authors: Osaki Y, Yokohama A, Saito A, Tahara K, Yanagisawa K, Ogawa Y, Ishizaki T, Mitsui T, Koiso H, Takizawa M, Uchiumi H, Saitoh T, Handa H, Murakami H, Tsukamoto N, Nojima Y
PLoS ONE, 2013-11-29;8(11):e81722.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Blood stem cell fate regulation by Delta-1-mediated rewiring of IL-6 paracrine signaling.
Authors: Csaszar, Elizabet, Wang, Weijia, Usenko, Tatiana, Qiao, Wenlian, Delaney, Colleen, Bernstein, Irwin D, Zandstra, Peter W
Blood, 2013-11-15;123(5):650-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Epigenetic regulation of NANOG by miR-302 cluster-MBD2 completes induced pluripotent stem cell reprogramming.
Authors: Lee M, Prasain N, Chae H, Kim Y, Mantel C, Yoder M, Broxmeyer H
Stem Cells, 2013-04-01;31(4):666-81.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neurokinin-1 receptor signalling impacts bone marrow repopulation efficiency.
Authors: Berger A, Frelin C, Shah D, Benveniste P, Herrington R, Gerard N, Zuniga-Pflucker J, Iscove N, Paige C
PLoS ONE, 2013-03-14;8(3):U630.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Authors: Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J
Nature, 2012-07-26;487(7408):505-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors.
Authors: Niwa A, Heike T, Umeda K
PLoS ONE, 2011-07-27;6(7):e22261.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases.
Authors: Ye L, Chang JC, Lin C, Sun X, Yu J, Kan YW
Proc. Natl. Acad. Sci. U.S.A., 2009-05-29;106(24):9826-30.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Isolation and characterization of CD146+ multipotent mesenchymal stromal cells.
Authors: Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M
Exp. Hematol., 2008-05-27;36(8):1035-46.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Refreezing of cord blood hematopoietic stem cells for allogenic transplantation: in vitro and in vivo validation of a clinical phase I/II protocol in European and Italian Good Manufacturing Practice conditions.
Authors: Gunetti M, Ferrero I, Rustichelli D, Berger M, Gammaitoni L, Timeus F, Piacibello W, Aglietta M, Fagioli F
Exp. Hematol., 2008-02-01;36(2):235-43.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Thrombopoietin enhances generation of CD34+ cells from human embryonic stem cells.
Authors: Srivastava AS, Nedelcu E, Esmaeli-Azad B, Mishra R, Carrier E
Stem Cells, 2007-03-22;25(6):1456-61.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Long-lasting in vitro hematopoiesis derived from primate embryonic stem cells.
Authors: Hiroyama T, Miharada K, Aoki N, Fujioka T, Sudo K, Danjo I, Nagasawa T, Nakamura Y
Exp. Hematol., 2006-06-01;34(6):760-9.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
Tal1/Scl gene transduction using a lentiviral vector stimulates highly efficient hematopoietic cell differentiation from common marmoset (Callithrix jacchus) embryonic stem cells.
Authors: Kurita R, Sasaki E, Yokoo T, Hiroyama T, Takasugi K, Imoto H, Izawa K, Dong Y, Hashiguchi T, Soda Y, Maeda T, Suehiro Y, Tanioka Y, Nakazaki Y, Tani K
Stem Cells, 2006-05-25;24(9):2014-22.
Species: Primate - Callitrix jacchus (Common Marmoset)
Sample Types: Whole Cells
Applications: Bioassay -
Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector.
Authors: Kahl CA, Tarantal AF, Lee CI, Jimenez DF, Choi C, Pepper K, Petersen D, Fletcher MD, Leapley AC, Fisher J, Burns TS, Ultsch MN, Dorey FJ, Kohn DB
Exp. Hematol., 2006-03-01;34(3):369-81.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
The reduction of in vitro radiation-induced Fas-related apoptosis in CD34+ progenitor cells by SCF, FLT-3 ligand, TPO, and IL-3 in combination resulted in CD34+ cell proliferation and differentiation.
Authors: Drouet M, 49153, Mathieu J, Grenier N, Multon E, Sotto JJ, Herodin F
Circulating NOD1 Activators and Hematopoietic NOD1 Contribute to Metabolic Inflammation and Insulin Resistance, 1999-01-01;17(5):273-85.
Species: Primate - Papio anubis (Olive Baboon)
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
What are the differences between Recombinant Human Flt-3 Ligand/FLT3L Protein (Catalog # 308-FK), Recombinant Human Flt-3 Ligand/FLT3L Protein (Catalog # 308-FKN), and Recombinant Human Flt-3 Ligand/FLT3L Protein, CF (Catalog # 308-FKE)?
Recombinant Human Flt-3 Ligand/FLT3L Protein (Catalog # 308-FK) and Recombinant Human Flt-3 Ligand/FLT3L Protein (Catalog # 308-FKN) share the same protein sequence, Thr27-Pro185 of Accession # AAA17999.1 but Catalog # 308-FK was expressed in Sf 21 baculovirus cells and Catalog # 308-FKN was produced in mammalian NS0 cells. Both of these proteins are glycosylated and available bottled with BSA or without BSA (Carrier-Free). Recombinant Human Flt-3 Ligand/FLT3L Protein, CF (Catalog # 308-FKE) was produced in an E. Coli expression system and has a sequence of (Thr27-Ala181, Accession # P49771.1), but may also contain an N-terminal Methionine followed by the expected sequence. In addition to being slightly shorter compared to 308-FKN and 308-FK, there is one difference in amino acid at position 72. Accession # AAA17999.1 has an Alanine at position 72, while Accession # P49771.1 has a Glycine in that position. All three versions of Recombinant Human Flt-3 Ligand/FLT3L proteins are routinely tested and have the same activity range in our QC testing assay: Measured in a cell proliferation assay using BaF3 mouse pro‑B cells transfected with mouse Flt-3. The ED50 for this effect is 0.2-1 ng/mL. R&D Systems also offers two GMP versions of this protein, an E. coli-derived animal-free version, Recombinant Human Flt-3 Ligand/FLT3L GMP Protein, CF (Catalog # 308E-GMP), and an Sf-21 baculovirus-derived version, Recombinant Human Flt-3 Ligand GMP Protein, CF (Catalog # 308-GMP). For information on the GMP proteins, we recommend consulting the product-specific pages for each protein. To determine which of these three proteins is most suitable for an application, we would recommend checking the Citations tab on the product-specific page.
Reviews for Recombinant Human Flt-3 Ligand/FLT3L Protein
Average Rating: 5 (Based on 3 Reviews)
Have you used Recombinant Human Flt-3 Ligand/FLT3L Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: