Recombinant Human KGF/FGF-7 Protein
Recombinant Human KGF/FGF-7 Protein Summary
Product Specifications
Cys32-Thr194, with an N-terminal Met
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
251-KG
| Formulation | Lyophilized from a 0.2 μm filtered solution in MOPS, Na2SO4 and EDTA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
251-KG/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in MOPS, Na2SO4 and EDTA. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
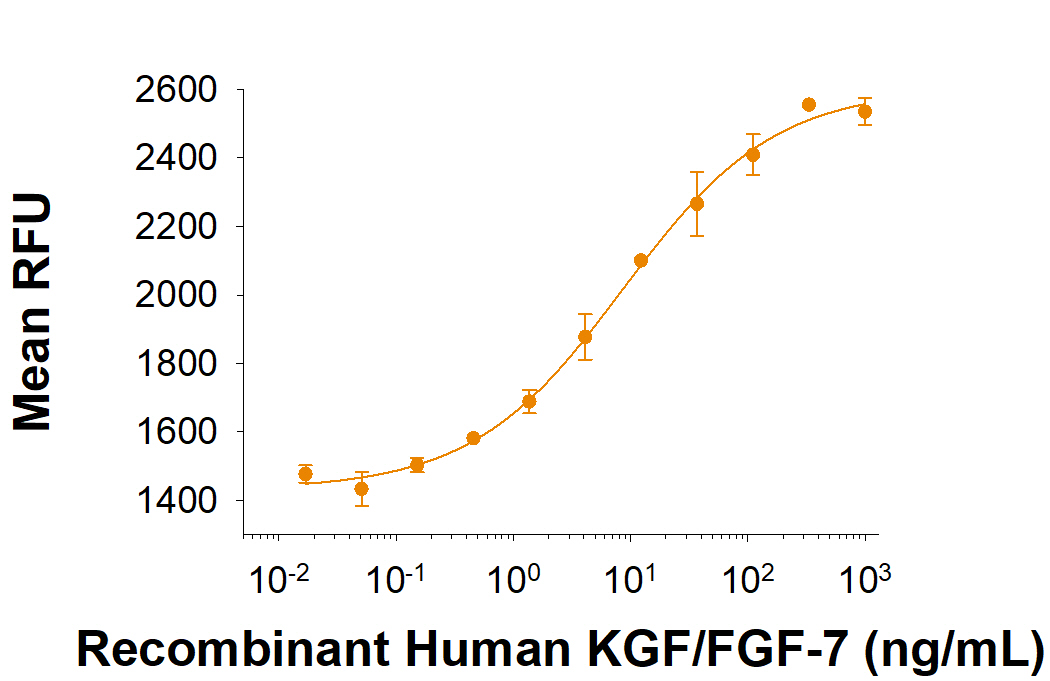 View Larger
View Larger
Recombinant Human KGF/FGF-7 (Catalog # 251-KG) stimulates cell proliferation of 4MBr-5 rhesus monkey epithelial cells. The ED50 for this effect is 6-60 ng/mL.
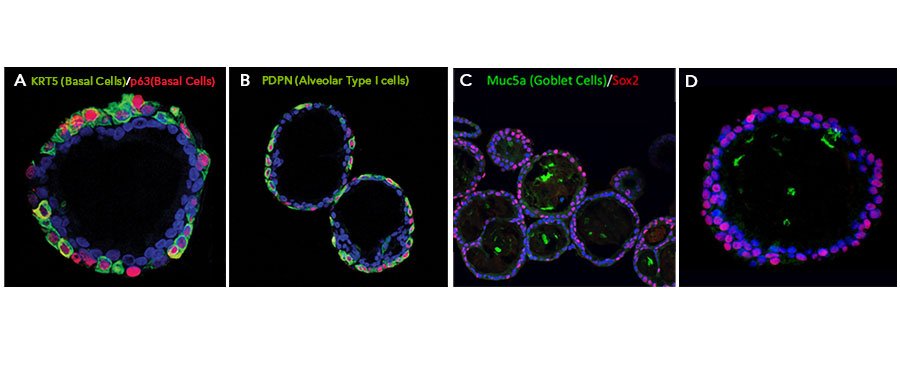 View Larger
View Larger
Adult stem cells isolated from human lung biopsy tissue were embedded in Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and cultured for 20-60 days in lung organoid expansion medium, which includes Recombinant Human KGF/FGF-7 (Catalog # 251-KG), Recombinant Human FGF-10 (345-FG), Recombinant Human Noggin (6057-NG), and Recombinant Human R-Spondin 1 (4645-RS), along with the other reagents listed in the lung organoid expansion medium recipe in the lung organoid culture protocol. Lung organoids were able to differentiate and exhibit markers for various cell types of the lung. Lung organoids were stained with (A) a Rabbit Anti-Human Cytokeratin 5 (KRT5) Monoclonal Antibody (Novus Biologicals, Catalog # NB110-56916; green) and a Goat Anti-Human p63/TP73L Polyclonal Antibody (AF1916; red) to visualize basal cells, (B) a Hamster Anti-Mouse Podoplanin (PDPN) Monoclonal Antibody (Novus Biologicals, Catalog # NB600-1015; green) to visualize alveolar type I cells and a Goat Anti-Human p63/TP73L Polyclonal Antibody (AF1916; red) to visualize basal cells, and (C, D) a Mouse Anti-MUC5AC Monoclonal Antibody (Novus Biologicals, Catalog # NBP2-15196; green) to visualize Goblet cells and a Mouse Anti-Human/Mouse/Rat SOX2 Monoclonal Antibody (MAB2018; red). All samples were counterstained with DAPI (5748; blue).
Reconstitution Calculator
Background: KGF/FGF-7
KGF (keratinocyte growth factor), also known as FGF-7 (fibroblast growth factor-7), is one of 22 known members of the mouse FGF family of secreted proteins that plays a key role in development, morphogenesis, angiogenesis, wound healing, and tumorigenesis (1-4). KGF expression is restricted to cells of mesenchymal origin. When secreted, it acts as a paracrine growth factor for nearby epithelial cells (1). KGF speeds wound healing by being dramatically upregulated in response to damage to skin or internal structures that results in high local concentrations of inflammatory mediators such as IL-1 and TNF-alpha. (2, 5). KGF promotes cell migration and invasion, and mediates melanocyte transfer to keratinocytes upon UVB radiation (6, 7). It has been used ectopically to avoid chemotherapy-induced oral mucositis in patients with hematological malignancies (1). Deletion of KGF affects kidney development, producing abnormally small ureteric buds and fewer nephrons (8). It also impedes hair follicle differentiation (9). The 194 amino acid (aa) KGF precursor contains a 31 aa signal sequence and, like all other FGFs, an ~120 aa beta -trefoil scaffold that includes receptor- and heparin-binding sites. KGF signals only through the IIIb splice form of the tyrosine kinase receptor, FGF R2 (FGF R2-IIIb/KGF R) (10). Receptor dimerization requires an octameric or larger heparin or heparin sulfate proteoglycan (11). FGF-10, also called KGF2, shares 51% aa identity and similar function to KGF, but shows more limited expression than KGF and uses an additional receptor, FGF R2-IIIc (12). Following receptor engagement, KGF is typically degraded, while FGF-10 is recycled (12). Mature human KGF, which is active across species, shares 98% aa sequence identity with bovine, equine, ovine and canine, 96% with mouse and porcine, and 92% with rat KGF, respectively.
- Finch, P.W. and J.S. Rubin (2006) J. Natl. Cancer Inst. 98:812.
- Werner, S. et al. (2007) J. Invest. Dermatol. 127:998.
- Werner, S. (1998) Cytokine Growth Factor Rev. 9:153.
- Mason, I.J. et al. (1994) Mech. Dev. 45:15.
- Geer, D.J. et al. (2005) Am. J. Pathol. 167:1575.
- Niu, J. et al. (2007) J. Biol. Chem. 282:6001.
- Cardinali, G. et al. (2005) J. Invest. Dermatol. 125:1190.
- Qiao, J. et al. (1999) Development 126:547.
- Guo, L. et al. (1996) Genes Dev. 10:165.
- de Georgi, V. et al. (2007) Dermatol. Clin. 25:477.
- Hsu, Y-R. et al. (1999) Biochemistry 38:2523.
- Belleudi, F. et al. (2007) Traffic 8:1854.
Citations for Recombinant Human KGF/FGF-7 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
84
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A keratinocyte-adipocyte signaling loop is reprogrammed by loss of BTG3 to augment skin carcinogenesis
Authors: Cheng, YC;Acedera, JD;Li, YJ;Shieh, SY;
Cell death and differentiation
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A distal lung organoid model to study interstitial lung disease, viral infection and human lung development
Authors: Matkovic Leko, I;Schneider, RT;Thimraj, TA;Schrode, N;Beitler, D;Liu, HY;Beaumont, K;Chen, YW;Snoeck, HW;
Nature protocols
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Opposing roles for TGF?- and BMP-signaling during nascent alveolar differentiation in the developing human lung
Authors: Frum, T;Hsu, PP;Hein, RFC;Conchola, AS;Zhang, CJ;Utter, OR;Anand, A;Zhang, Y;Clark, SG;Glass, I;Sexton, JZ;Spence, JR;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoids
Applications: Differentiation, Differentiation -
Generation and expansion of transitional lung organoids from human pluripotent stem cells
Authors: IM Leko, N Schrode, J Torres, M Pezet, TA Thimraj, KG Beaumont, HW Snoeck
bioRxiv : the preprint server for biology, 2023-02-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Epigenetic and transcriptional regulations prime cell fate before division during human pluripotent stem cell differentiation
Authors: P Madrigal, S Deng, Y Feng, S Militi, KJ Goh, R Nibhani, R Grandy, A Osnato, D Ortmann, S Brown, S Pauklin
Nature Communications, 2023-01-25;14(1):405.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
iPSCs derived from esophageal atresia patients reveal SOX2 dysregulation at the anterior foregut stage
Authors: S Raad, A David, M Sagniez, B Paré, Z Orfi, NA Dumont, MA Smith, C Faure
Disease Models & Mechanisms, 2022-11-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Organoids capture tissue-specific innate lymphoid cell development in mice and humans
Authors: GM Jowett, E Read, LB Roberts, D Coman, M Vilà Gonzá, T Zabinski, U Niazi, R Reis, TJ Trieu, D Danovi, E Gentleman, L Vallier, MA Curtis, GM Lord, JF Neves
Cell Reports, 2022-08-30;40(9):111281.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Exposure to e-cigarette vapor extract induces vocal fold epithelial injury and triggers intense mucosal remodeling
Authors: V Lungova, K Wendt, SL Thibeault
Oncogene, 2022-08-23;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages
Authors: HR Seo, HJ Han, Y Lee, YW Noh, SJ Cho, JH Kim
International Journal of Molecular Sciences, 2022-08-16;23(16):.
Species: Human
Sample Types: Organoids
Applications: Cell Culture -
CRISPR interference interrogation of COPD GWAS genes reveals the functional significance of desmoplakin in iPSC-derived alveolar epithelial cells
Authors: RB Werder, T Liu, KM Abo, J Lindstrom-, C Villacorta, J Huang, A Hinds, N Boyer, E Bullitt, M Liesa, EK Silverman, DN Kotton, MH Cho, X Zhou, AA Wilson
Oncogene, 2022-07-13;8(28):eabo6566.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of Isogenic hiPSCs with Targeted Edits at Multiple Intronic SNPs to Study the Effects of the Type 2 Diabetes Associated KCNQ1 Locus in American Indians
Authors: AK Nair, M Traurig, JR Sutherland, YL Muller, ED Grellinger, L Saporito, RG Nelson, C Bogardus, LJ Baier
Cells, 2022-04-25;11(9):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Microwell bag culture for large-scale production of homogeneous islet-like clusters
Authors: R Suenaga, S Konagaya, J Yamaura, R Ito, S Tanaka, Y Ishizaki, T Toyoda
Scientific Reports, 2022-03-25;12(1):5221.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
An HNF1alpha truncation associated with maturity-onset diabetes of the young impairs pancreatic progenitor differentiation by antagonizing HNF1beta function
Authors: AM Cujba, ME Alvarez-Fa, S Pedraza-Ar, A Laddach, MH Shepherd, AT Hattersley, FM Watt, R Sancho
Cell Reports, 2022-03-01;38(9):110425.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Intracameral Microimaging of Maturation of Human iPSC Derivatives into Islet Endocrine Cells
Authors: K Zhao, Y Shi, J Yu, L Yu, A Mael, Y Li, A Kolton, T Joyce, J Odorico, PO Berggren, SN Yang
Cell Transplantation, 2022-01-01;31(0):9636897211066.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells
Authors: JJ Kathiriya, C Wang, M Zhou, A Brumwell, M Cassandras, CJ Le Saux, M Cohen, KD Alysandrat, B Wang, P Wolters, M Matthay, DN Kotton, HA Chapman, T Peng
Nature Cell Biology, 2021-12-30;0(0):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Sequence logic at enhancers governs a dual mechanism of endodermal organ fate induction by FOXA pioneer factors
Authors: RJ Geusz, A Wang, DK Lam, NK Vinckier, KD Alysandrat, DA Roberts, J Wang, S Kefalopoul, A Ramirez, Y Qiu, J Chiou, KJ Gaulton, B Ren, DN Kotton, M Sander
Nature Communications, 2021-11-17;12(1):6636.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Dysfunctional lactate metabolism in human alveolar type II cells from idiopathic pulmonary fibrosis lung explant tissue
Authors: DA Newton, RG Lottes, RM Ryan, DD Spyropoulo, JE Baatz
Respiratory Research, 2021-10-28;22(1):278.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection
Authors: AV Parent, G Faleo, J Chavez, M Saxton, DI Berrios, NR Kerper, Q Tang, M Hebrok
Cell Reports, 2021-08-17;36(7):109538.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Development of a quantitative prediction algorithm for target organ-specific similarity of human pluripotent stem cell-derived organoids and cells
Authors: MO Lee, SG Lee, CR Jung, YS Son, JW Ryu, KB Jung, JH Ahn, JH Oh, HA Lee, JH Lim, J Kim, I Jang, J Choi, J Jung, K Park, B Lee, DS Kim, MY Son, HS Cho
Nature Communications, 2021-07-23;12(1):4492.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Histone deacetylase inhibitor panobinostat induces antitumor activity in epithelioid sarcoma and rhabdoid tumor by growth factor receptor modulation
Authors: AC Harttrampf, MEM da Costa, A Renoult, E Daudigeos-, B Geoerger
BMC Cancer, 2021-07-20;21(1):833.
Species: Human
Sample Types: Whole Cells
Applications: Stimulation -
The in vitro multilineage differentiation and maturation of lung and airway cells from human pluripotent stem cell-derived lung progenitors in 3D
Authors: AL Rodrigues, HY Liu, YW Chen, M Porotto, A Moscona, HW Snoeck
Nature Protocols, 2021-03-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PGC7 promotes tumor oncogenic dedifferentiation through remodeling DNA methylation pattern for key developmental transcription factors
Authors: Q Yan, Y Zhang, X Fang, B Liu, TL Wong, L Gong, S Liu, D Yu, M Liu, L Jiang, X Wang, T Wei, Y Jia, L Li, L Sun, Y Tang, N Zhou, YF Yuan, Y Li, S Ma, XY Guan
Cell Death and Differentiation, 2021-01-26;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CXCR4 Regulates Temporal Differentiation via PRC1 Complex in Organogenesis of Epithelial Glands
Authors: J Kim, SW Lee, K Park
International Journal of Molecular Sciences, 2021-01-10;22(2):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Heat-Not-Burn cigarette induces oxidative stress response in primary rat alveolar epithelial cells
Authors: Y Ito, K Oshinden, N Kutsuzawa, C Kohno, S Isaki, K Yokoyama, T Sato, M Tanaka, K Asano
PLoS ONE, 2020-11-25;15(11):e0242789.
Species: Rat
Sample Types: Whole Cells
Applications: Cell Culture -
SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response
Authors: J Huang, AJ Hume, KM Abo, RB Werder, C Villacorta, KD Alysandrat, ML Beermann, C Simone-Roa, J Lindstrom-, J Olejnik, EL Suder, E Bullitt, A Hinds, A Sharma, M Bosmann, R Wang, F Hawkins, EJ Burks, M Saeed, AA Wilson, E Mühlberger, DN Kotton
Cell Stem Cell, 2020-09-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Modeling COVID-19 with Human Pluripotent Stem Cell-Derived Cells Reveals Synergistic Effects of Anti-inflammatory Macrophages with ACE2 Inhibition Against SARS-CoV-2
Authors: F Duan, L Guo, L Yang, Y Han, A Thakur, BE Nilsson-Pa, P Wang, Z Zhang, CY Ma, X Zhou, T Han, T Zhang, X Wang, D Xu, X Duan, J Xiang, HF Tse, C Liao, W Luo, FP Huang, YW Chen, T Evans, RE Schwartz, B tenOever, DD Ho, S Chen, Q Lian, HJ Chen
Res Sq, 2020-09-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Organoids Model Transcriptional Hallmarks of Oncogenic KRAS Activation in Lung Epithelial Progenitor Cells
Authors: AFM Dost, AL Moye, M Vedaie, LM Tran, E Fung, D Heinze, C Villacorta, J Huang, R Hekman, JH Kwan, BC Blum, SM Louie, SP Rowbotham, J Sainz de A, ME Piper, PJ Bhetariya, RT Bronson, A Emili, G Mostoslavs, GA Fishbein, WD Wallace, K Krysan, SM Dubinett, J Yanagawa, DN Kotton, CF Kim
Cell Stem Cell, 2020-09-04;27(4):663-678.e8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
SARS-CoV-2 Infection of Pluripotent Stem Cell-derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response
Authors: J Huang, AJ Hume, KM Abo, RB Werder, C Villacorta, KD Alysandrat, ML Beermann, C Simone-Roa, J Olejnik, EL Suder, E Bullitt, A Hinds, A Sharma, M Bosmann, R Wang, F Hawkins, EJ Burks, M Saeed, AA Wilson, E Mühlberger, DN Kotton
bioRxiv, 2020-08-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Antagonism of interferon signaling by fibroblast growth factors promotes viral replication
Authors: L Maddaluno, C Urwyler, T Rauschendo, M Meyer, D Stefanova, R Spörri, M Wietecha, L Ferrarese, D Stoycheva, D Bender, N Li, G Strittmatt, K Nasirujjam, HD Beer, P Staeheli, E Hildt, A Oxenius, S Werner
EMBO Mol Med, 2020-07-27;0(0):e11793.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
RSK2 Maintains Adult Estrogen Homeostasis by Inhibiting ERK1/2-Mediated Degradation of Estrogen Receptor Alpha
Authors: KA Ludwik, ZM Sandusky, KM Stauffer, Y Li, KL Boyd, GA O'Doherty, TP Stricker, DA Lannigan
Cell Rep, 2020-07-21;32(3):107931.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids
Authors: L Yang, Y Han, BE Nilsson-Pa, V Gupta, P Wang, X Duan, X Tang, J Zhu, Z Zhao, F Jaffré, T Zhang, TW Kim, O Harschnitz, D Redmond, S Houghton, C Liu, A Naji, G Ciceri, S Guttikonda, Y Bram, DT Nguyen, M Cioffi, V Chandar, DA Hoagland, Y Huang, J Xiang, H Wang, D Lyden, A Borczuk, HJ Chen, L Studer, FC Pan, DD Ho, BR tenOever, T Evans, RE Schwartz, S Chen
Cell Stem Cell, 2020-06-19;27(1):125-136.e7.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
LSD1-mediated enhancer silencing attenuates retinoic acid signalling during pancreatic endocrine cell development
Authors: NK Vinckier, NA Patel, RJ Geusz, A Wang, J Wang, I Matta, AR Harrington, M Wortham, N Wetton, J Wang, US Jhala, MG Rosenfeld, CW Benner, HP Shih, M Sander
Nat Commun, 2020-04-29;11(1):2082.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A hepatocyte differentiation model reveals two subtypes of liver cancer with different oncofetal properties and therapeutic targets
Authors: M Liu, Q Yan, Y Sun, Y Nam, L Hu, JH Loong, Q Ouyang, Y Zhang, HL Li, FE Kong, L Li, Y Li, MM Li, W Cheng, LX Jiang, S Fang, XD Yang, JQ Mo, YF Gong, YQ Tang, Y Li, YF Yuan, NF Ma, G Lin, S Ma, JG Wang, XY Guan
Proc. Natl. Acad. Sci. U.S.A., 2020-03-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids
Authors: L Yang, Y Han, BE Nilsson-Pa, V Gupta, P Wang, X Duan, X Tang, J Zhu, Z Zhao, F Jaffré, T Zhang, TW Kim, O Harschnitz, D Redmond, S Houghton, C Liu, A Naji, G Ciceri, S Guttikonda, Y Bram, DT Nguyen, M Cioffi, V Chandar, DA Hoagland, Y Huang, J Xiang, H Wang, D Lyden, A Borczuk, HJ Chen, L Studer, FC Pan, DD Ho, BR tenOever, T Evans, RE Schwartz, S Chen
Cell Stem Cell, 2020;27(1):125-136.e7.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells
Authors: A Jacob, M Vedaie, DA Roberts, DC Thomas, C Villacorta, KD Alysandrat, F Hawkins, DN Kotton
Nat Protoc, 2019-11-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Use of Modified Clostridium perfringens Enterotoxin Fragments for Claudin Targeting in Liver and Skin Cells
Authors: LS Beier, J Rossa, S Woodhouse, S Bergmann, HB Kramer, J Protze, M Eichner, A Piontek, S Vidal-Y-Sy, JM Brandner, G Krause, N Zitzmann, J Piontek
Int J Mol Sci, 2019-09-26;20(19):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human induced pluripotent stem cell-derived vocal fold mucosa mimics development and responses to smoke exposure
Authors: V Lungova, X Chen, Z Wang, C Kendziorsk, SL Thibeault
Nat Commun, 2019-09-24;10(1):4161.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
KLF4 prevents epithelial to mesenchymal transition in human corneal epithelial cells via endogenous TGF- β2 suppression
Authors: S Fujimoto, R Hayashi, S Hara, Y Sasamoto, J Harrington, M Tsujikawa, K Nishida
Regen Ther, 2019-09-12;11(0):249-257.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FOXA2 Is Required for Enhancer Priming during Pancreatic Differentiation
Authors: K Lee, H Cho, RW Rickert, QV Li, J Pulecio, CS Leslie, D Huangfu
Cell Rep, 2019-07-09;28(2):382-393.e7.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Point mutations in the PDX1 transactivation domain impair human ?-cell development and function
Authors: X Wang, M Sterr, Ansarullah, I Burtscher, A Böttcher, J Beckenbaue, J Siehler, T Meitinger, HU Häring, H Staiger, FM Cernilogar, G Schotta, M Irmler, J Beckers, CVE Wright, M Bakhti, H Lickert
Mol Metab, 2019-03-20;0(0):.
Species: Human
Sample Types: Whole Cells
-
Generation of lung organoids from human pluripotent stem cells in vitro
Authors: AJ Miller, BR Dye, D Ferrer-Tor, DR Hill, AW Overeem, LD Shea, JR Spence
Nat Protoc, 2019-02-01;14(2):518-540.
Species: Human
Sample Types: Spheroids
Applications: Bioassay -
An integrated chromatin accessibility and transcriptome landscape of human pre-implantation embryos
Authors: L Liu, L Leng, C Liu, C Lu, Y Yuan, L Wu, F Gong, S Zhang, X Wei, M Wang, L Zhao, L Hu, J Wang, H Yang, S Zhu, F Chen, G Lu, Z Shang, G Lin
Nat Commun, 2019-01-21;10(1):364.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pancreatic Cell Fate Determination Relies on Notch Ligand Trafficking by NFIA
Authors: MA Scavuzzo, J Chmielowie, D Yang, K Wamble, LS Chaboub, L Duraine, B Tepe, SM Glasgow, BR Arenkiel, C Brou, B Deneen, M Borowiak
Cell Rep, 2018-12-26;25(13):3811-3827.e7.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
3D Modeling of Esophageal Development using Human PSC-Derived Basal Progenitors Reveals a Critical Role for Notch Signaling
Authors: Y Zhang, Y Yang, M Jiang, SX Huang, W Zhang, D Al Alam, S Danopoulos, M Mori, YW Chen, R Balasubram, SM Chuva de S, C Serra, M Bialecka, E Kim, S Lin, ALR Toste de C, PN Riccio, WV Cardoso, X Zhang, HW Snoeck, J Que
Cell Stem Cell, 2018-09-20;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation
Authors: JP Ng-Blichfe, A Schrik, RK Kortekaas, JA Noordhoek, IH Heijink, PS Hiemstra, J Stolk, M Königshoff, R Gosens
EBioMedicine, 2018-09-17;36(0):461-474.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Voltage-dependent Ca2+ channels promote branching morphogenesis of salivary glands by patterning differential growth
Authors: JM Kim, S Choi, SW Lee, K Park
Sci Rep, 2018-05-15;8(1):7566.
Species: Mouse
Sample Types: Complex Sample Type
Applications: Bioassay -
IL-13 induces periostin and eotaxin expression in human primary alveolar epithelial cells: Comparison with paired airway epithelial cells
Authors: Y Ito, R Al Mubarak, N Roberts, K Correll, W Janssen, J Finigan, R Mishra, HW Chu
PLoS ONE, 2018-04-19;13(4):e0196256.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Decoding the dynamic DNA methylation and hydroxymethylation landscapes in endodermal lineage intermediates during pancreatic differentiation of hESC
Authors: J Li, X Wu, Y Zhou, M Lee, L Guo, W Han, W Mo, WM Cao, D Sun, R Xie, Y Huang
Nucleic Acids Res., 2018-04-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Beta-Catenin maintains lung epithelial progenitors after lung specification
Authors: EJ Ostrin, DR Little, KN Gerner-Mau, EA Sumner, R Ríos-Corzo, E Ambrosio, SE Holt, NR Forcioli-C, H Akiyama, SM Hanash, S Kimura, SXL Huang, J Chen
Development, 2018-03-09;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Genome-wide analysis of PDX1 target genes in human pancreatic progenitors
Authors: X Wang, M Sterr, I Burtscher, S Chen, A Hieronimus, F Machicao, H Staiger, HU Häring, G Lederer, T Meitinger, FM Cernilogar, G Schotta, M Irmler, J Beckers, M Hrab? de A, M Ray, CVE Wright, M Bakhti, H Lickert
Mol Metab, 2018-01-31;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of a human embryonic stem cell line expressing tetrameric Zoanthus sp. green fluorescent protein: NERCe002-A-1
Authors: X Duan, M Xie, Y Peng, L Hu, J Yu, S Zeng, Y Wang, G Lu, G Lin, Y Sun
Stem Cell Res, 2018-01-31;28(0):6-10.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Functional vascularized lung grafts for lung bioengineering
Authors: NV Dorrello, BA Guenthart, JD O'Neill, J Kim, K Cunningham, YW Chen, M Biscotti, T Swayne, HM Wobma, SXL Huang, HW Snoeck, M Bacchetta, G Vunjak-Nov
Sci Adv, 2017-08-30;3(8):e1700521.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Small molecule AT7867 proliferates PDX1-expressing pancreatic progenitor cells derived from human pluripotent stem cells
Authors: A Kimura, T Toyoda, Y Nishi, M Nasu, A Ohta, K Osafune
Stem Cell Res, 2017-08-17;24(0):61-68.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor
Authors: M Yoshihara, Y Sasamoto, R Hayashi, Y Ishikawa, M Tsujikawa, Y Hayashizak, M Itoh, H Kawaji, K Nishida
Sci Rep, 2017-06-06;7(1):2845.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Ex vivo analysis of the contribution of FGF10(+) cells to airway smooth muscle cell formation during early lung development
Authors: E El Agha, V Kheirollah, A Moiseenko, W Seeger, S Bellusci
Dev. Dyn., 2017-06-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Phosphorylation of NEUROG3 Links Endocrine Differentiation to the Cell Cycle in Pancreatic Progenitors
Authors: NAJ Krentz, D van Hoof, Z Li, A Watanabe, M Tang, C Nian, MS German, FC Lynn
Dev. Cell, 2017-04-24;41(2):129-142.e6.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling
Authors: KB McCauley, F Hawkins, M Serra, DC Thomas, A Jacob, DN Kotton
Cell Stem Cell, 2017-03-30;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Radioprotective effects of Keratinocyte Growth Factor-1 against irradiation-induced salivary gland hypofunction
Authors: JS Choi, HS Shin, HY An, YM Kim, JY Lim
Oncotarget, 2017-02-21;8(8):13496-13508.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Defined three-dimensional culture conditions mediate efficient induction of definitive endoderm lineage from human umbilical cord Wharton's jelly mesenchymal stem cells
Stem Cell Res Ther, 2016-11-16;7(1):165.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Hyaluronan Does Not Regulate Human Epidermal Keratinocyte Proliferation and Differentiation
Authors: J Malaisse, V Pendaries, F Hontoir, V De Glas, D Van Vlaend, M Simon, C Lambert de, Y Poumay, B Flamion
J. Biol. Chem, 2015-12-01;291(12):6347-58.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Unliganded fibroblast growth factor receptor 1 forms density-independent dimers.
Authors: Comps-Agrar L, Dunshee D, Eaton D, Sonoda J
J Biol Chem, 2015-08-13;290(40):24166-77.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
DNA methylation directs functional maturation of pancreatic beta cells.
Authors: Dhawan S, Tschen S, Zeng C, Guo T, Hebrok M, Matveyenko A, Bhushan A
J Clin Invest, 2015-06-22;125(7):2851-60.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The role of HGF/MET and FGF/FGFR in fibroblast-derived growth stimulation and lapatinib-resistance of esophageal squamous cell carcinoma.
Authors: Saito S, Morishima K, Ui T, Hoshino H, Matsubara D, Ishikawa S, Aburatani H, Fukayama M, Hosoya Y, Sata N, Lefor A, Yasuda Y, Niki T
BMC Cancer, 2015-02-25;15(0):82.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Efficient generation of functional CFTR-expressing airway epithelial cells from human pluripotent stem cells.
Authors: Wong, Amy P, Chin, Stephani, Xia, Sunny, Garner, Jodi, Bear, Christin, Rossant, Janet
Nat Protoc, 2015-02-05;10(3):363-81.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells.
Authors: Huang S, Green M, de Carvalho A, Mumau M, Chen Y, D'Souza S, Snoeck H
Nat Protoc, 2015-02-05;10(3):413-25.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human marrow stromal cells downsize the stem cell fraction of lung cancers by fibroblast growth factor 10.
Authors: Kanehira M, Kikuchi T, Santoso A, Tode N, Hirano T, Ohkouchi S, Tamada T, Sugiura H, Harigae H, Ichinose M
Mol Cell Biol, 2014-05-27;34(15):2848-56.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers.
Authors: Lam A, Freedman B, Morizane R, Lerou P, Valerius M, Bonventre J
J Am Soc Nephrol, 2013-12-19;25(6):1211-25.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Efficient generation of lung and airway epithelial cells from human pluripotent stem cells.
Authors: Huang, Sarah X, Islam, Mohammad, O'Neill, John, Hu, Zheng, Yang, Yong-Gua, Chen, Ya-Wen, Mumau, Melanie, Green, Michael, Vunjak-Novakovic, Gordana, Bhattacharya, Jahar, Snoeck, Hans-Wil
Nat Biotechnol, 2013-12-01;32(1):84-91.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
Molecular mechanism of SSR128129E, an extracellularly acting, small-molecule, allosteric inhibitor of FGF receptor signaling.
Authors: Herbert C, Schieborr U, Saxena K, Juraszek J, De Smet F, Alcouffe C, Bianciotto M, Saladino G, Sibrac D, Kudlinzki D, Sreeramulu S, Brown A, Rigon P, Herault J, Lassalle G, Blundell T, Rousseau F, Gils A, Schymkowitz J, Tompa P, Herbert J, Carmeliet P, Gervasio F, Schwalbe H, Bono F
Cancer Cell, 2013-04-15;23(4):489-501.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A stimulation-dependent alternate core promoter links lymphotoxin alpha expression with TGF-beta1 and fibroblast growth factor-7 signaling in primary human T cells.
Authors: Yokley B, Selby S, Posch P
J Immunol, 2013-04-01;190(9):4573-84.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions.
Authors: Brafman D, Phung C, Kumar N, Willert K
Cell Death Differ, 2012-11-16;20(3):369-81.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells.
Authors: Prabakar K, Dominguez-Bendala J, Molano R, Pileggi A, Villate S, Ricordi C, Inverardi L
Cell Transplant, 2011-12-21;21(6):1321-39.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer.
Authors: Tomlinson DC, Knowles MA
Am. J. Pathol., 2010-10-01;177(5):2379-86.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Platelet-derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression.
Authors: Yamamoto S, Fukumoto E, Yoshizaki K, Iwamoto T, Yamada A, Tanaka K, Suzuki H, Aizawa S, Arakaki M, Yuasa K, Oka K, Chai Y, Nonaka K, Fukumoto S
J. Biol. Chem., 2008-06-17;283(34):23139-49.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation.
Authors: Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP
J. Biol. Chem., 2008-01-28;283(14):9308-17.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis.
Authors: Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock JM, Elkin M, Vlodavsky I, Hoffman MP
Development, 2007-10-24;134(23):4177-86.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis.
Authors: Sa SM, Valdez PA, Wu J
J. Immunol., 2007-02-15;178(4):2229-40.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro.
Authors: Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ
Am. J. Respir. Cell Mol. Biol., 2007-01-25;36(6):661-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neuritogenic activity of chondroitin/dermatan sulfate hybrid chains of embryonic pig brain and their mimicry from shark liver. Involvement of the pleiotrophin and hepatocyte growth factor signaling pathways.
Authors: Li F, Shetty AK, Sugahara K
J. Biol. Chem., 2006-12-04;282(5):2956-66.
Species: Fish - Prionace glauca (Blue Shark)
Sample Types: Peptide
Applications: Bioassay -
FGF2 posttranscriptionally down-regulates expression of SDF1 in bone marrow stromal cells through FGFR1 IIIc.
Authors: Nakayama T, Mutsuga N, Tosato G
Blood, 2006-10-31;109(4):1363-72.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Development of an in vitro pancreatic tissue model to study regulation of islet neogenesis associated protein expression.
Authors: Petropavlovskaia M, Makhlin J, Sampalis J, Rosenberg L
J. Endocrinol., 2006-10-01;191(1):65-81.
Species: Hamster
Sample Types: Whole Cells
Applications: Bioassay -
The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair.
Authors: Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S
Oncogene, 2005-08-11;24(34):5269-77.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Keratinocyte-fibroblast paracrine interaction: the effects of substrate and culture condition.
Authors: Witte RP, Kao WJ
Biomaterials, 2005-06-01;26(17):3673-82.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis.
Authors: Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP
Development, 2005-02-16;132(6):1223-34.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human KGF/FGF-7 Protein
Average Rating: 5 (Based on 1 Review)
Have you used Recombinant Human KGF/FGF-7 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:

