Recombinant Human LIF Protein Summary
Product Specifications
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
7734-LF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
7734-LF/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Reconstitution Calculator
Background: LIF
LIF (leukemia inhibitory factor) is a widely expressed, highly and variably glycosylated, 32-62 kDa, monomeric, pleiotropic cytokine in the IL-6 family of helical cytokines (1-4). The first exon encoding the signal sequence is alternately spliced, resulting in LIF-D, LIF-M, and LIF-T mRNAs that produce secreted, extracellular matrix-associated, and intracellular forms, respectively (5). LIF-D and LIF-M mRNAs produce identical 180 amino acid (aa) mature sequences (5). Mature human LIF (180 aa) shares 78%, 82%, 91%, 88 and 87% aa sequence identity with mouse, rat, canine, bovine, and porcine LIF, respectively. The LIF receptor is a heterodimer of a type I transmembrane ligand-binding subunit, LIFR (gp190), and the type I transmembrane signal transducing subunit, gp130, signaling especially through STAT3 and JAK kinases (3, 4, 6). Gp130 and members of the LIFR family also mediate the biological effects of Oncostatin M, Cardiotrophin-1, Galectin-10, CNTF, IL-6, IL-11, and IL-27 (3, 6). A soluble LIFR has been reported in the mouse (7). Depending on the cells and their context, LIF either opposes or favors differentiation (3, 8). LIF produced by the uterine endometrium supports successful implantation of the embryo, promotes proliferation and maintenance of pluripotency in embryonic stem cells, and favors proliferation of progenitor cell types such as hematopoietic stem cells (3, 6, 8). However, excess LIF blocks differentiation of embryoid bodies, indicating the importance of LIF regulation (3, 6). LIF is produced by CD4 + T cells in response to activation, and is required by the thymic epithelium to support T cell maturation (3, 4). LIF expression is up-regulated by neuronal injury, and promotes motor neuron survival and oligodendrocyte myelination (3, 4, 9). LIF is produced by the adrenal cortex and likely enhances its production of cortisol and aldosterone (10). LIF can function as an autocrine growth factor in some pancreatic cancers, but induces differentiation in the myeloid leukemic cell line M1 (2, 11). Tumor LIF can also induce formation of immunosuppressive tumor-associated macrophages (12). LIF promotes endometrial remodeling and differentiation of adipocytes and cardiac smooth muscle cells (3, 4). It promotes regulatory T cell and inhibits Th17 cell differentiation, thus promoting tolerance, down-regulating inflammation, and contributing to immune tolerance during pregnancy and in the nervous system (3, 4, 6, 8).
- Gough, N.M. et al. (1988) Proc. Natl. Acad. Sci. USA 85:2623.
- Moreau, J.F. et al. (1988) Nature 336:690.
- Trouillas, M. et al. (2009) Eur. Cytokine Netw. 20:51.
- Metcalfe, S.M. (2011) Genes Immun. 12:157.
- Voyle, R.B. et al. (1999) Exp. Cell Res. 249:199.
- Cheng, J.G. et al. (2001) Proc. Natl. Acad. Sci. USA 98:8680.
- Tomida, M. et al. (1993) FEBS lett. 334:193.
- Paiva, P. et al. (2009) Cytokine Growth Factor Rev. 20:319.
- Slaets, H. et al. (2010) Trends Mol. Med. 16:493.
- Bamberger, A.M. et al. (2000) Mol. Cell. Endocrinol. 162:145.
- Kamohara, H. et al. (2007) Int. J. Oncol. 30:977.
- Duluc, D. et al. (2007) Blood 110:4319.
Citations for Recombinant Human LIF Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
30
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Engineered Interleukin-6-derived cytokines recruit artificial receptor complexes and disclose CNTF signaling via the OSMR
Authors: Rafii, P;Cruz, PR;Ettich, J;Seibel, C;Padrini, G;Wittich, C;Lang, A;Petzsch, P;Köhrer, K;Moll, JM;Floss, DM;Scheller, J;
The Journal of biological chemistry
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TGF-?-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer
Authors: Zhang, F;Yan, Y;Cao, X;Guo, C;Wang, K;Lv, S;
Cell death & disease
Species: Human hepegivirus
Sample Types: Whole Cells
Applications: Bioassay -
Nonspecific Inhibition of IL6 Family Cytokine Signalling by Soluble gp130
Authors: Widjaja, AA;Cook, SA;
International journal of molecular sciences
-
Cynomolgus monkey embryo model captures gastrulation and early pregnancy
Authors: J Li, Q Zhu, J Cao, Y Liu, Y Lu, Y Sun, Q Li, Y Huang, S Shang, X Bian, C Li, L Zhang, Y Wang, Y Nie, J Fu, W Li, MA Mazid, Y Jiang, W Jia, X Wang, Y Sun, MA Esteban, Q Sun, F Zhou, Z Liu
Cell Stem Cell, 2023-04-06;30(4):362-377.e7.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
Leukemia inhibitory factor is a therapeutic target for renal interstitial fibrosis
Authors: S Xu, X Yang, Q Chen, Z Liu, Y Chen, X Yao, A Xiao, J Tian, L Xie, M Zhou, Z Hu, F Zhu, X Xu, F Hou, J Nie
EBioMedicine, 2022-11-04;86(0):104312.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Bioassay -
The balance between NANOG and SOX17 mediated by TET proteins regulates specification of human primordial germ cell fate
Authors: Z Li, F Fang, Y Long, Q Zhao, X Wang, Z Ye, T Meng, X Gu, W Xiang, C Xiong, H Li
Cell & bioscience, 2022-11-04;12(1):181.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CoSpar identifies early cell fate biases from single-cell transcriptomic and lineage information
Authors: SW Wang, MJ Herriges, K Hurley, DN Kotton, AM Klein
Nature Biotechnology, 2022-02-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pleiotropic, Unique and Shared Responses Elicited by IL-6 Family Cytokines in Human Vascular Endothelial Cells
Authors: M Lindkvist, MM Zegeye, M Grenegård, LU Ljungberg
International Journal of Molecular Sciences, 2022-01-27;23(3):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PCK1 regulates neuroendocrine differentiation in a positive feedback loop of LIF/ZBTB46 signalling in castration-resistant prostate cancer
Authors: YC Wen, CL Liu, HL Yeh, WH Chen, KC Jiang, VTN Tram, M Hsiao, J Huang, WY Chen, YN Liu
British Journal of Cancer, 2021-11-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of human blastocyst-like structures from pluripotent stem cells
Authors: Y Fan, Z Min, S Alsolami, Z Ma, E Zhang, W Chen, K Zhong, W Pei, X Kang, P Zhang, Y Wang, Y Zhang, L Zhan, H Zhu, C An, R Li, J Qiao, T Tan, M Li, Y Yu
Cell Discovery, 2021-09-07;7(1):81.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Leukemia inhibitory factor is involved in the pathogenesis of NSCLC through activation of the STAT3 signaling pathway
Authors: H Wang, S Si, M Jiang, L Chen, K Huang, W Yu
Oncology Letters, 2021-07-14;22(3):663.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
HDAC inhibitors induce LIFR expression and promote a dormancy phenotype in breast cancer
Authors: ME Clements, L Holtslande, C Edwards, V Todd, SDR Dooyema, K Bullock, K Bergdorf, CA Zahnow, RM Connolly, RW Johnson
Oncogene, 2021-07-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The histone demethylase KDM2B regulates human primordial germ cell-like cells specification
Authors: W Yuan, Z Yao, V Veerapandi, X Yang, X Wang, D Chen, L Ma, C Li, Y Zheng, F Luo, XY Zhao
International journal of biological sciences, 2021-01-01;17(2):527-538.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
EGFR-upregulated LIFR promotes SUCLG2-dependent castration resistance and neuroendocrine differentiation of prostate cancer
Authors: SR Lin, YC Wen, HL Yeh, KC Jiang, WH Chen, N Mokgautsi, J Huang, WY Chen, YN Liu
Oncogene, 2020-09-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Analysis of multi-lineage gene expression dynamics during primordial germ cell induction from human induced pluripotent stem cells
Authors: F Fang, Z Li, Q Zhao, C Xiong, K Ni
Stem Cell Res Ther, 2020-03-04;11(1):100.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Supplementation of vitamin C promotes early germ cell specification from human embryonic stem cells
Authors: Z Li, F Fang, Q Zhao, H Li, C Xiong
Stem Cell Res Ther, 2019-11-15;10(1):324.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
EC359-A first-in-class small molecule inhibitor for targeting oncogenic LIFR signaling in triple negative breast cancer
Authors: S Viswanadha, Y Luo, GR Sareddy, B Santhamma, M Zhou, M Li, S Ma, R Sonavane, UP Pratap, KA Altwegg, X Li, A Chang, AC Riveros, KV Dileep, KYJ Zhang, X Pan, R Murali, M Bajda, GV Raj, AJ Brenner, V Manthati, MK Rao, RR Tekmal, HB Nair, KJ Nickisch, RK Vadlamudi
Mol. Cancer Ther., 2019-05-29;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Surface Plasmon Resonance -
STAT3 signaling stimulates miR-21 expression in bovine cumulus cells during in vitro oocyte maturation
Authors: A Tscherner, AC Brown, L Stalker, J Kao, I Dufort, MA Sirard, J LaMarre
Sci Rep, 2018-08-01;8(1):11527.
Species: Bovine
Sample Types: Whole Cells
Applications: Bioassay -
The transcription factor Vezf1 represses the expression of the antiangiogenic factor d2 in endothelial cells
Authors: L Alabdi, M He, Q Yang, AB Norvil, H Gowher
J. Biol. Chem., 2018-05-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
LIFR increases the release of sEng via the up-regulation of MMP14 expression in PE
Authors: H Li, J Yao, X Chang, J Wu, T Duan, K Wang
Reproduction, 2018-01-23;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
miR-124 promotes proliferation and differentiation of neuronal stem cells through inactivating Notch pathway
Authors: S Jiao, Y Liu, Y Yao, J Teng
Cell Biosci, 2017-12-11;7(0):68.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Combining Injectable Plasma Scaffold with Mesenchymal Stem/Stromal Cells for Repairing Infarct Cavity after Ischemic Stroke
Authors: H Zhang, F Sun, J Wang, L Xie, C Yang, M Pan, B Shao, GY Yang, SH Yang, Q ZhuGe, K Jin
Aging Dis, 2017-04-01;8(2):203-214.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Increased susceptibility to a? toxicity in neuronal cultures derived from familial Azheimer's disease (PSEN1-A246E) induced pluripotent stem cells
Authors: Enrique Armijo
Neurosci. Lett, 2016-12-26;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow
Nat Cell Biol, 2016-09-19;18(10):1078-1089.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Novel Small-Molecule Inhibitor Targeting the IL-6 Receptor beta Subunit, Glycoprotein 130.
Authors: Hong S, Choi J, Lee S, Park Y, Park K, Lee J, Kim J, Gajulapati V, Goo J, Singh S, Lee K, Kim Y, Im S, Ahn S, Rose-John S, Heo T, Choi Y
J Immunol, 2015-05-29;195(1):237-45.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Targeted Knockdown of RNA-Binding Protein TIAR for Promoting Self-Renewal and Attenuating Differentiation of Mouse Embryonic Stem Cells.
Authors: Geng, Zhe, Li, Ping, Tan, Li, Song, Houyan
Stem Cells Int, 2015-03-31;2015(0):657325.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of IL-17A responses in human airway smooth muscle cells by Oncostatin M.
Authors: Kwofie K, Scott M, Rodrigues R, Guerette J, Radford K, Nair P, Richards C
Respir Res, 2015-02-07;16(0):14.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling.
Authors: Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, Limame R, Mestdagh P, Vandesompele J, Vanhove C, Maynard D, Lehuede C, Muller C, Valet P, Gespach C, Bracke M, Cocquyt V, Denys H, De Wever O
Cancer Res, 2014-09-24;74(23):6806-19.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice.
Authors: Kondo T, Funayama M, Tsukita K, Hotta A, Yasuda A, Nori S, Kaneko S, Nakamura M, Takahashi R, Okano H, Yamanaka S, Inoue H
Stem Cell Reports, 2014-06-26;3(2):242-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The amino acid exchange R28E in ciliary neurotrophic factor (CNTF) abrogates interleukin-6 receptor-dependent but retains CNTF receptor-dependent signaling via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor (LIFR).
Authors: Wagener E, Aurich M, Aparicio-Siegmund S, Floss D, Garbers C, Breusing K, Rabe B, Schwanbeck R, Grotzinger J, Rose-John S, Scheller J
J Biol Chem, 2014-05-06;289(26):18442-50.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human LIF Protein
Average Rating: 4.3 (Based on 3 Reviews)
Have you used Recombinant Human LIF Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
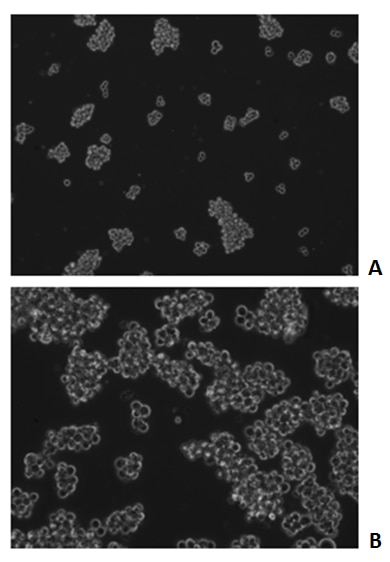
Growth of MA-11 spheroids in serum-free condition in the absence (A) or presence (B) of Recombinant Human LIF (#7734-LF) after 72 hours. Medium: RPMI + 2% B-27 supplement + 1% L-glutamine + 1% Pen/Strep.