TGF-beta Pan Specific Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
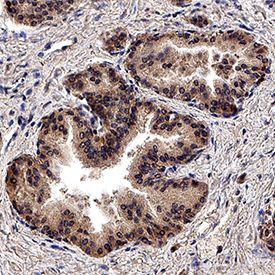
TGF-beta Pan Specific in Human Prostate Tissue. TGF-beta was detected in immersion fixed paraffin-embedded sections of human prostate tissue using Rabbit Anti-TGF-beta Pan Specific Polyclonal Antibody (Catalog # AB-100-NA) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC003). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in epitjhelial cells. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
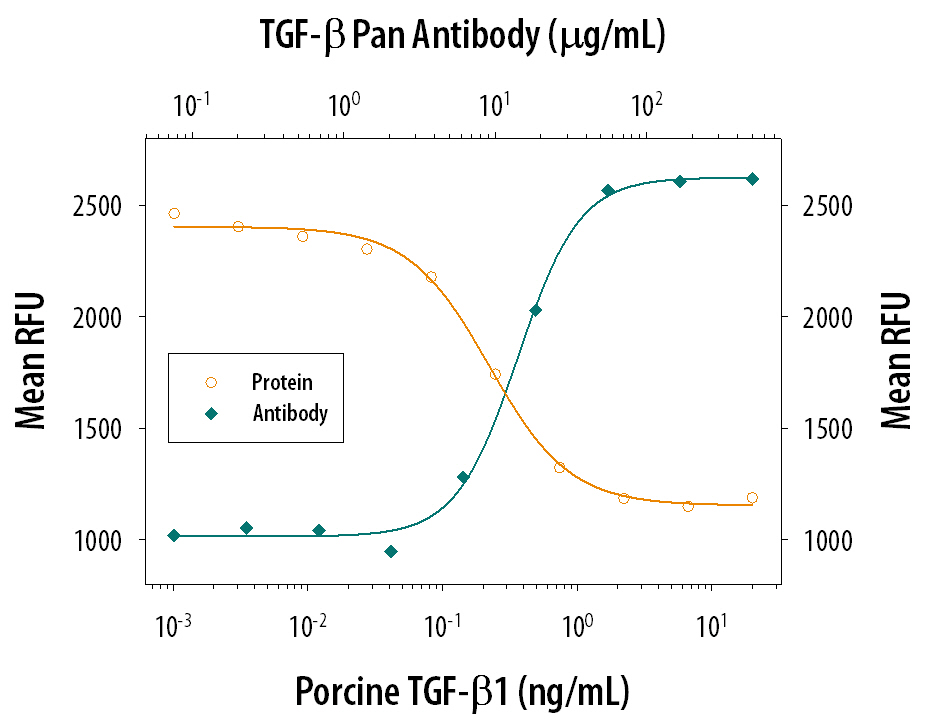
TGF‑ beta 1 Inhibition of IL‑4-dependent Cell Proliferation and Neutralization by TGF‑ beta Antibody. Porcine TGF-beta 1 (Catalog # 101-B1) inhibits Recombinant Mouse IL-4 (Catalog # 404-ML) induced proliferation in the HT-2 mouse T cell line in a dose-dependent manner (orange line). Inhibition of Recombinant Mouse IL-4 (7.5 ng/mL) activity elicited by Porcine TGF-beta 1 (1 ng/mL) is neutralized (green line) by increasing concentrations of TGF-beta Pan Specific Polyclonal Antibody (Catalog # AB-100-NA). The ND50range is 5-30 µg/mL.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TGF-beta
TGF-beta 1 (transforming growth factor beta 1) is one of three closely related mammalian members of the large TGF-beta superfamily that share a characteristic cystine knot structure. TGF-beta 1, -2 and -3 are highly pleiotropic cytokines that are proposed to act as cellular switches that regulate processes such as immune function, proliferation and epithelial-mesenchymal transition. Each TGF-beta isoform has some non-redundant functions; for TGF-beta 1, mice with targeted deletion show defects in hematopoiesis and endothelial differentiation, and die of overwhelming inflammation. Human TGF-beta 1 cDNA encodes a 390 amino acid (aa) precursor that contains a 29 aa signal peptide and a 361 aa proprotein. A furin-like convertase processes the proprotein to generate an N-terminal 249 aa latency-associated peptide (LAP) and a C-terminal 112 aa mature TGF- beta 1. Disulfide-linked homodimers of LAP and TGF-beta 1 remain non-covalently associated after secretion, forming the small latent TGF-beta 1 complex. Covalent linkage of LAP to one of three latent TGF-beta binding proteins (LTBPs) creates a large latent complex that may interact with the extracellular matrix. TGF-beta is activated from latency by pathways that include actions of the protease plasmin, matrix metalloproteases, thrombospondin 1 and a subset of integrins. Mature human TGF-beta 1 shares 100% aa identity with pig, dog and cow TGF-beta 1, and 99% aa identity with mouse, rat and horse TGF-beta 1. It demonstrates cross-species activity. TGF-beta 1 signaling begins with high-affinity binding to a type II ser/thr kinase receptor termed TGF-beta RII. This receptor then phosphorylates and activates a second ser/thr kinase receptor, TGF-beta RI (also called activin receptor-like kinase (ALK) -5), or alternatively, ALK‑1. This complex phosphorylates and activates Smad proteins that regulate transcription. Contributions of the accessory receptors betaglycan (also known as TGF-beta RIII) and endoglin, or use of Smad-independent signaling pathways, allow for disparate actions observed in response to TGF-beta in different contexts.
Product Datasheets
Citations for TGF-beta Pan Specific Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
56
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Expression of TGF-beta and MMP-2 in hereditary gingival fibromatosis epithelial cells. A possible contribution of the epithelium to its pathogenesis
Authors: NM Kamal, MA Hamouda, N Abdelgawad
Journal of oral biology and craniofacial research, 2022-08-14;12(5):617-622.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of miR-155 Promotes TGF-beta Mediated Suppression of HIV Release in the Cervical Epithelial Cells
Authors: J Gokavi, S Sadawarte, A Shelke, U Kulkarni-K, M Thakar, V Saxena
Viruses, 2021-11-12;13(11):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-&beta loaded exosome enhances ischemic wound healing in vitro and in vivo
Authors: A Shi, J Li, X Qiu, M Sabbah, S Boroumand, TC Huang, C Zhao, A Terzic, A Behfar, SL Moran
Theranostics, 2021-04-30;11(13):6616-6631.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging
Authors: YE Choi, MJ Song, M Hara, K Imanaka-Yo, DH Lee, JH Chung, ST Lee
Int J Mol Sci, 2020-11-18;21(22):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-&beta in the Secretome of Irradiated Peripheral Blood Mononuclear Cells Supports In Vitro Osteoclastogenesis
Authors: L Panahipour, Z Kargarpour, M Laggner, M Mildner, HJ Ankersmit, R Gruber
Int J Mol Sci, 2020-11-13;21(22):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Roxatidine inhibits fibrosis by inhibiting NF?kappaB and MAPK signaling in macrophages sensing breast implant surface materials
Authors: L Ji, T Wang, L Tian, H Song, M Gao
Mol Med Rep, 2019-11-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Cervical cancer cells produce TGF-?1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-?1
Authors: R García-Roc, A Monroy-Gar, J Hernández-, B Weiss-Stei, V Gutiérrez-, M Del Carmen, LR Ávila-Ibar, CA Don-López, DB Torres-Pin, M de Lourdes
Cytokine, 2018-10-06;118(0):71-79.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
DNA methylation affects metastasis of renal cancer and is associated with TGF-?/RUNX3 inhibition
Authors: J Zheng, Y Mei, P Xiang, G Zhai, N Zhao, C Xu, M Liu, Z Pan, K Tang, D Jia
Cancer Cell Int., 2018-04-10;18(0):56.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Cardiac macrophages promote diastolic dysfunction
Authors: M Hulsmans, HB Sager, JD Roh, M Valero-Muñ, NE Houstis, Y Iwamoto, Y Sun, RM Wilson, G Wojtkiewic, B Tricot, MT Osborne, J Hung, C Vinegoni, K Naxerova, DE Sosnovik, MR Zile, AD Bradshaw, R Liao, A Tawakol, R Weissleder, A Rosenzweig, FK Swirski, F Sam, M Nahrendorf
J. Exp. Med., 2018-01-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
The IL-2/Anti-IL-2 Complex Attenuates Cardiac Ischaemia-Reperfusion Injury Through Expansion of Regulatory T Cells
Authors: J Xiao, K Yu, M Li, C Xiong, Y Wei, Q Zeng
Cell. Physiol. Biochem., 2017-12-07;44(5):1810-1827.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Naive CD4(+) T Cells Carrying a TLR2 Agonist Overcome TGF-beta-Mediated Tumor Immune Evasion.
Authors: Ibrahim M, Scozzi D, Toth K, Ponti D, Kreisel D, Menna C, De Falco E, D'Andrilli A, Rendina E, Calogero A, Krupnick A, Gelman A
J Immunol, 2017-12-06;200(2):847-856.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
TGF-? contamination of purified recombinant GDF15
Authors: OE Olsen, A Skjærvik, BF Størdal, A Sundan, T Holien
PLoS ONE, 2017-11-21;12(11):e0187349.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Nonmyocyte ERK1/2 signaling contributes to load-induced cardiomyopathy in Marfan mice
Authors: R Rouf, EG MacFarlane, E Takimoto, R Chaudhary, V Nagpal, PP Rainer, JG Bindman, EE Gerber, D Bedja, C Schiefer, KL Miller, G Zhu, L Myers, N Amat-Alarc, DI Lee, N Koitabashi, DP Judge, DA Kass, HC Dietz
JCI Insight, 2017-08-03;2(15):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-? Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation
Authors: N Selvamurug, Z He, D Rifkin, B Dabovic, NC Partridge
Stem Cells Int, 2017-04-23;2017(0):2450327.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ST2/IL-33-Dependent Microglial Response Limits Acute Ischemic Brain Injury
Authors: Y Yang, H Liu, H Zhang, Q Ye, J Wang, B Yang, L Mao, W Zhu, RK Leak, B Xiao, B Lu, J Chen, X Hu
J. Neurosci., 2017-04-07;0(0):.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Neutralization -
Endoplasmic Reticulum Oxidative Stress Triggers Tgf-Beta-Dependent Muscle Dysfunction by Accelerating Ascorbic Acid Turnover
Authors: D Pozzer, M Favellato, M Bolis, RW Invernizzi, F Solagna, B Blaauw, E Zito
Sci Rep, 2017-01-20;7(0):40993.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
MXRA5 is a TGF-?1-regulated human protein with anti-inflammatory and anti-fibrotic properties
J Cell Mol Med, 2016-09-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Lymphatic vessels regulate immune microenvironments in human and murine melanoma
J Clin Invest, 2016-08-15;0(0):.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
TGF-? Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions
Authors: X Chen, DL Rateri, DA Howatt, A Balakrishn, JJ Moorleghen, LA Cassis, A Daugherty
PLoS ONE, 2016-04-22;11(4):e0153811.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Osmotic Induction of Angiogenic Growth Factor Expression in Human Retinal Pigment Epithelial Cells.
Authors: Veltmann M, Hollborn M, Reichenbach A, Wiedemann P, Kohen L, Bringmann A
PLoS ONE, 2016-01-22;11(1):e0147312.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Stearoyl-CoA desaturase 1 and paracrine diffusible signals have a major role in the promotion of breast cancer cell migration induced by cancer-associated fibroblasts.
Authors: Angelucci C, Maulucci G, Colabianchi A, Iacopino F, D'Alessio A, Maiorana A, Palmieri V, Papi M, De Spirito M, Di Leone A, Masetti R, Sica G
Br J Cancer, 2015-04-16;112(10):1675-86.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence.
Authors: Gonzalez-Valdes I, Hidalgo I, Bujarrabal A, Lara-Pezzi E, Padron-Barthe L, Garcia-Pavia P, Gomez-del Arco P, Redondo J, Ruiz-Cabello J, Jimenez-Borreguero L, Enriquez J, de la Pompa J, Hidalgo A, Gonzalez S
Nat Commun, 2015-03-09;6(0):6473.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Identification of genetic loci that control mammary tumor susceptibility through the host microenvironment.
Authors: Zhang P, Lo A, Huang Y, Huang G, Liang G, Mott J, Karpen G, Blakely E, Bissell M, Barcellos-Hoff M, Snijders A, Mao J
Sci Rep, 2015-03-09;5(0):8919.
Species: Mouse
Sample Types: Whole Tissue
Applications: Blocking -
CD4(+)Foxp3(+) Tregs protect against innate immune cell-mediated fulminant hepatitis in mice.
Authors: Hou X, Song J, Su J, Huang D, Gao W, Yan J, Shen J
Mol Immunol, 2014-10-12;63(2):420-7.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Emdogain-regulated gene expression in palatal fibroblasts requires TGF-betaRI kinase signaling.
Authors: Stahli A, Bosshardt D, Sculean A, Gruber R
PLoS ONE, 2014-09-08;9(9):e105672.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Platelets provoke distinct dynamics of immune responses by differentially regulating CD4+ T-cell proliferation.
Authors: Zhu L, Huang Z, Stalesen R, Hansson G, Li N
J Thromb Haemost, 2014-06-27;12(7):1156-65.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Expression of versican V3 by arterial smooth muscle cells alters tumor growth factor beta (TGFbeta)-, epidermal growth factor (EGF)-, and nuclear factor kappaB (NFkappaB)-dependent signaling pathways, creating a microenvironment that resists monocyte adhesion.
Authors: Kang I, Yoon D, Braun K, Wight T
J Biol Chem, 2014-04-09;289(22):15393-404.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Loss of Dab2 expression in breast cancer cells impairs their ability to deplete TGF-beta and induce Tregs development via TGF-beta.
Authors: Xu S, Zhu J, Wu Z
PLoS ONE, 2014-03-17;9(3):e91709.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF.
Authors: Bernard M, Dieude M, Yang B, Hamelin K, Underwood K, Hebert M
Autophagy, 2014-01-01;10(12):2193-207.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells.
Authors: Kim M, Koo T, Yan J, Lee E, Han K, Jeong J, Ro H, Kim B, Jo S, Oh K, Surh C, Ahn C, Yang J
J Am Soc Nephrol, 2013-07-05;24(10):1529-36.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade.
Authors: Olson B, Jankowska-Gan E, Becker J, Vignali D, Burlingham W, McNeel D
J Immunol, 2012-11-14;189(12):5590-601.
Species: Human
Sample Types: In Vivo
Applications: Bioassay -
Toll-like Receptor 2 (TLR2), Transforming Growth Factor-beta, Hyaluronan (HA), and Receptor for HA-mediated Motility (RHAMM) Are Required for Surfactant Protein A-stimulated Macrophage Chemotaxis.
Authors: Foley J, Lam D, Jiang H, Liao J, Cheong N, McDevitt T, Zaman A, Wright J, Savani R
J Biol Chem, 2012-09-04;287(44):37406-19.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Transglutaminase 2 cross-linking activity is linked to invadopodia formation and cartilage breakdown in arthritis.
Authors: Lauzier A, Charbonneau M, Paquette M, Harper K, Dubois CM
Arthritis Res. Ther., 2012-07-04;14(4):R159.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche.
Authors: Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H
Cell, 2011-11-23;147(5):1146-58.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease.
Authors: Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y
J. Clin. Invest., 2011-10-17;121(11):4548-66.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
IL-33 induces IL-9 production in human CD4+ T cells and basophils.
Authors: Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK
PLoS ONE, 2011-07-06;6(7):e21695.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut.
Authors: Suzuki K, Maruya M, Kawamoto S
Immunity, 2010-07-23;33(1):71-83.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture.
Authors: King VL, Lin AY, Kristo F, Anderson TJ, Ahluwalia N, Hardy GJ, Owens AP, Howatt DA, Shen D, Tager AM, Luster AD, Daugherty A, Gerszten RE
Circulation, 2009-01-12;119(3):426-35.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4.
Authors: Chase JC, Celli J, Bosio CM
Infect. Immun., 2008-11-03;77(1):180-95.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Molecular mechanisms of TGFbeta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506.
Authors: Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W
J. Immunol., 2008-08-15;181(4):2831-45.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Local administration of interleukin-11 ameliorates intestinal radiation injury in rats.
Authors: Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M
Cancer Res., 2007-10-01;67(19):9501-6.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease.
Authors: Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG
Am. J. Respir. Crit. Care Med., 2007-08-29;176(10):974-82.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling.
Authors: Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ
Am. J. Respir. Cell Mol. Biol., 2007-08-02;38(1):95-104.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Advanced glycation end products decrease mesangial cell MMP-7: a role in matrix accumulation in diabetic nephropathy?
Authors: McLennan SV, Kelly DJ, Schache M, Waltham M, Dy V, Langham RG, Yue DK, Gilbert RE
Kidney Int., 2007-06-06;72(4):481-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states.
Authors: Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC
Nat. Med., 2007-01-21;13(2):204-10.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Increase in transforming growth factor-beta in the brain during infection is related to fever, not depression of spontaneous motor activity.
Authors: Matsumura S, Shibakusa T, Fujikawa T, Yamada H, Inoue K, Fushiki T
Neuroscience, 2006-12-06;144(3):1133-40.
Species: Rat
Sample Types: CSF
Applications: Neutralization -
Uptake of host cell transforming growth factor-beta by Trypanosoma cruzi amastigotes in cardiomyocytes: potential role in parasite cycle completion.
Authors: Waghabi MC, Keramidas M, Bailly S, Degrave W, Mendonca-Lima L, Soeiro Mde N, Meirelles Mde N, Paciornik S, Araujo-Jorge TC, Feige JJ
Am. J. Pathol., 2005-10-01;167(4):993-1003.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Electron Microscopy, ICC, IHC-P, Neutralization -
Nitric oxide induces TIMP-1 expression by activating the transforming growth factor beta-Smad signaling pathway.
Authors: Akool el-S, Doller A, Muller R, Gutwein P, Xin C, Huwiler A, Pfeilschifter J, Eberhardt W
J. Biol. Chem., 2005-09-23;280(47):39403-16.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Thrombospondin plays a vital role in the immune privilege of the eye.
Authors: Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW
Invest. Ophthalmol. Vis. Sci., 2005-03-01;46(3):908-19.
Species: Mink, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Neuronal TGF-beta1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice.
Authors: Mesples B, Fontaine RH, Lelievre V, Launay JM, Gressens P
Neurobiol. Dis., 2005-02-01;18(1):193-205.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity.
Authors: Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, Myllymaa S, Kaivo-Oja N, Lankinen H, Mottershead DG, Groome NP, Ritvos O
Biol. Reprod., 2004-05-05;71(3):732-9.
Species: Bovine, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10.
Authors: Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA
J. Immunol., 2004-05-01;172(9):5213-21.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates.
Authors: Torrealba JR, Katayama M, Fechner JH, Jankowska-Gan E, Kusaka S, Schultz JM, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ
J. Immunol., 2004-05-01;172(9):5753-64.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Neutralization -
Effect of weightbearing on bone formation during distraction osteogenesis.
Authors: Leung KS, Cheung WH, Yeung HY, Lee KM, Fung KP
Clin. Orthop. Relat. Res., 2004-02-01;0(419):251-7.
Species: Goat
Sample Types: Whole Tissue
Applications: IHC-P -
Connective tissue growth factor and its correlation to other growth factors in experimental granulation tissue.
Authors: Inkinen K, Wolff H, Lindroos P, Ahonen J
Connect. Tissue Res., 2003-01-01;44(1):19-29.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Estrogen inhibition of PTH-stimulated osteoclast formation and attachment in vitro: involvement of both PKA and PKC.
Authors: Liu BY, 2019, Wu PW, Bringhurst FR, Wang JT
141-147, 2002-02-01;143(2):627-35.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization
FAQs
-
Does TGF-beta Pan Specific Antibody, catalog # AB-100-NA, recognize active or latent TGF-beta (LAP)?
This antibody recognizes mature (active) TGF-beta. We have not tested for cross-reactivity to LAP (catalog # 246-LP) in our neutralization assay. Some cross-reactivity to latent TGF-beta may also be observed in western blot.
Reviews for TGF-beta Pan Specific Antibody
Average Rating: 4.3 (Based on 3 Reviews)
Have you used TGF-beta Pan Specific Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: