UDP-Azido-GalNAc Summary
Key Benefits
Learn more about Fluorescent Glycan Labeling and Detection

|
Formula |
C17H24N6O17P2 |
|
Molecular Weight |
646.35 Da |
|
Formulation |
1 mM provided in 20 mM Tris, pH 8.0 |
|
Stability & Storage |
Store the unopened product at < -20 °C. Good for 12 months from date of receipt. |
Incorporate or detect GalNAc without expensive, specialized equipment!
Applications
- For in vitro enzymatic incorporation of azido-sugars into specific, targeted glycans.
- Detect the presence or absence of GalNAc modifications.
- Monitoring O-glycans.
Key Features and Benefits
- Can be introduced to proteins and lipids via various GalNAc transferases.
- Can be conjugated to desired reporter molecules via click chemistry.
- Can be detected via Western blot, ELISA, and flow cytometry, depending on the type of reporter molecule.
- Contain the smallest possible orthogonal functional group.
- Has minimal side effect on target molecules.
- User-friendly.
For Details: Wu et al., (2015) Carbohydrate Res. 412:1-6
Related Reagents
Click Chemistry
Enzymes and Detection Reagents for UDP-Azido-GalNAc, ES103
|
GalNAc Transferases: All transfer GalNAc to Ser/Thr to initiate mucin-type glycosylation |
| GALNT2 |
| GALNTL1 |
| Polypeptide GalNAc Transferase 1/GALNT1 |
| Polypeptide GalNAc Transferase 10/GALNT10 |
| Polypeptide GalNAc Transferase 11/GALNT11 |
| Polypeptide GalNAc Transferase 13/GALNT13 |
| Polypeptide GalNAc Transferase 3/GALNT3 |
| Polypeptide GalNAc Transferase 4/GALNT4 |
| Polypeptide GalNAc Transferase 7/GALNT7 |
Schematic
|
Glycans are removed by enzyme treatment. Specific transferases can be used to incorporate Azido-GalNAc at suitable open positions. The incorporation Azido-GalNAc can then be detected using Biotinylated Alkyne in a click chemistry reaction. |
Sample Data
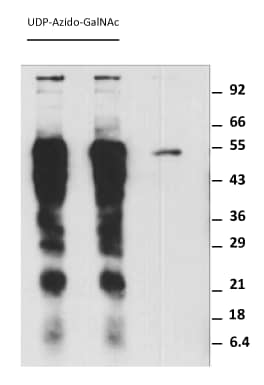
|
Labeling Asialofetuin with GALNT2. In each reaction, 5 µg of Asialofetuin (Sigma Aldrich), 0.5 nmol of UDP-Azido-GlcNAc, 1 µg rhGALNT2 and 1 µg of rE. faecalis O-Glycosidase was mixed in 50 µL of 25 mM HEPES supplemented with 10 mM of MnCl2 and 150 mM NaCl at pH 7.5. The reactions were incubated at 37°C for 60 minutes. The reactions were then conjugated, at room temperature for 30 minutes, with 1.0 nmol of Biotinylated Alkyne in the presence of 100 nmol of Ascorbic Acid and 5 nmol of CuCl2 for a final volume of 60 µL. The reactions were then separated with 12% SDS-PAGE and blotted to a nitrocellulose paper and detected with Streptavidin-HRP. |
Specifications
Product Datasheets
Assay Procedure
Sample Protocol for Labeling Glycoprotein with O-glycan Replacement
Protocols are guidelines. Parameters need to be optimized by end users.
Materials
- Protein Sample
- Assay Buffer: 25 mM HEPES, 150 mM NaCl, 10 mM MnCl2, pH 7.5.
- Recombinant Human GALNT2 (R&D Systems, Catalog # 7507-GT)
- Recombinant C. perfringens Neuraminidase (R&D Systems, Catalog # 5080-NM)
- Recombinant E. faecalis O-Glycosidase (R&D Systems, Catalog # 8886-GH)
- UDP-Azido-GalNAc (R&D Systems, Catalog # ES103)
- Biotinylated Alkyne (R&D Systems, Catalog # ES100)
- CuCl2, 1 mM in deionized water
- Ascorbic Acid, 20 mM in deionized water
- SDS-PAGE and Western blot reagents or equivalent
- TBST buffer: 25 mM Tris, 137 mM NaCl, 0.1% Tween-20, pH 7.5
- Streptavidin-HRP (R&D Systems, Catalog # DY998)
Assay Procedure
1. Prepare a reaction mixture by combining 5 µg Protein Sample, with 1 µg rhGALNT2, 0.2 µg rE. faecalis O-Glycosidase, 0.1 µg rcpNeuraminidase, 0.5 nmol UDP-Azido-GlcNAc in the Assay Buffer with the final volume of 25 µL.
2. Prepare negative controls according to step 1 but omit rhGALNT2 or UDP-Azido-GlcNAc.
3. Incubate all the reactions and controls at 37°C for one hour.
4. To each of the samples, add 5 µL of 1 mM CuCl2, 5 µL of 20 mM Ascorbic Acid, and 5 µL of 1 mM Biotinylated Alkyne. Mix with gentle tapping.
5. Incubate all samples at room temperature for 1 hour.
6. Separate the reactions and controls by SDS-PAGE.
7. Blot the gel to a nitrocellulose membrane.
8. Block the blot with 10% fat-free milk for 5 minutes.
9. Thoroughly wash the membrane with TBST buffer by changing buffer three times for a total of 45 minutes.
10. Incubate the blot with 25 ng/mL Streptavidin-HRP in 30 mL TBST buffer for 30 minutes.
11. Thoroughly wash the membrane with TBST buffer by changing buffer three times for a total of 45 minutes.
12. Detect with commercial ECL (Enhanced Chemiluminescence) reagents.
Final Assay Conditions Per Reaction
- UDP-Azido-GalNAc: 0.5 nmol
- rhGALNT2: 1 µg
- rcpNeuraminidase: 0.1 µg
- rE. faecalis O-Glycosidase : 0.2 µg
- Protein Sample: 5 µg
- Reaction volume: 25 µl
Click Chemistry Reaction Conditions Per Reaction
- CuCl2: 5 nmol
- Ascorbic Acid: 100 nmol
- Biotinylated Alkyne: 5 nmol
- Reaction volume: 40 µl
Citations for UDP-Azido-GalNAc
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
A universal glycoenzyme biosynthesis pipeline that enables efficient cell-free remodeling of glycans
Authors: T Jaroentome, YH Kwon, Y Liu, O Young, R Bhawal, JD Wilson, M Li, DG Chapla, KW Moremen, MC Jewett, D Mizrachi, MP DeLisa
Nature Communications, 2022-10-24;13(1):6325. 2022-10-24
-
Imaging specific cellular glycan structures using glycosyltransferases via click chemistry.
Authors: Wu Z, Person A, Anderson M, Burroughs B, Tatge T, Khatri K, Zou Y, Wang L, Geders T, Zaia J, Sackstein R
Glycobiology, 2018-02-01;0(0):. 2018-02-01
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for UDP-Azido-GalNAc
There are currently no reviews for this product. Be the first to review UDP-Azido-GalNAc and earn rewards!
Have you used UDP-Azido-GalNAc?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image