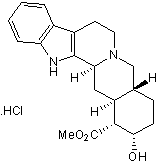
Yohimbine hydrochloride
Chemical Name: 17α-Hydroxyyohimban-16α-carboxylic acid methyl ester hydrochloride
Purity: ≥98%
Biological Activity
Yohimbine hydrochloride is a α2-adrenoceptor antagonist (pKi values are 8.52, 8.00 and 9.17 for human α2A, α2B and α2C receptors respectively).Diastereomer also available.
Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Additional Information
Background References
-
Comparison of the α-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine.
Doxey et al.
Naunyn Schmiedebergs Arch.Pharmacol., 1984;325:136 -
Yohimbine dimers exhibiting selectivity for the human α2c-adrenoceptor subtype.
Lalchandani et al.
J.Pharmacol.Exp.Ther., 2002;303:979 -
A re-evaluation of the role of α2-adrenoceptors in the anxiogenic effects of yohimbine, using the selective antagonist delequamine in the rat.
Redfern and Williams
Br.J.Pharmacol., 1995;116:2081 -
Yohimbine: a pharmacological probe for study of the α2-adrenoceptor.
Goldberg and Robertson
Pharmacol.Rev., 1987;35:143
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for Yohimbine hydrochloride
The citations listed below are publications that use Tocris products. Selected citations for Yohimbine hydrochloride include:
21 Citations: Showing 1 - 10
-
Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats.
Authors: Hirschberg Et al.
Elife 2017;6
-
Presynaptic GABAB receptors reduce transmission at parabrachial synapses in the lateral central amygdala by inhibiting N-type calcium channels.
Authors: Delaney and Crane
Sci Rep 2016;6:19255
-
Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats
Authors: Kastman Et al.
Neuropharmacology 2016;110:82
-
The Effects of Electrical and Optical Stimulation of Midbrain DArgic Neurons on Rat 50-kHz Ultrasonic Vocalizations.
Authors: Scardochio Et al.
Front Behav Neurosci 2015;9:331
-
A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health.
Authors: Soty Et al.
Psychopharmacology (Berl) 2015;4:106
-
Psychopharmacological characterisation of the successive negative contrast effect in rats.
Authors: Phelps Et al.
Proc Natl Acad Sci U S A 2015;232:2697
-
PTSD-like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation.
Authors: Gazarini Et al.
Int J Neuropsychopharmacol 2015;18
-
A Comparison of the Anorectic Effect and Safety of the Alpha2-Adrenoceptor Ligands guanf. and Yohimbine in Rats with Diet-Induced Obesity.
Authors: Dudek Et al.
PLoS One 2015;10:e0141327
-
The degree of acute descending control of spinal nociception in an area of primary hyperalgesia is dependent on the peripheral domain of afferent input.
Authors: Drake Et al.
J Physiol 2014;592:3611
-
Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat.
Authors: Ciccocioppo Et al.
Neuropsychopharmacology 2014;39:2601
-
Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats.
Authors: Costello Et al.
Neuropsychopharmacology 2014;39:1843
-
Dual effects of 5-HT(1a) receptor activation on breathing in neonatal mice.
Authors: Corcoran Et al.
Mol Metab 2014;34:51
-
Synergistic regulation of glutamatergic transmission by serotonin and NE reuptake inhibitors in prefrontal cortical neurons.
Authors: Yuen Et al.
J Biol Chem 2014;289:25177
-
Mechanism of ghrelin-induced gastric contractions in Suncus murinus (house musk shrew): involvement of intrinsic primary afferent neurons.
Authors: Mondal Et al.
PLoS One 2013;8:e60365
-
Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine.
Authors: Gozzi Et al.
Neuropsychopharmacology 2013;38:2120
-
Relaxin-3/RXFP3 system regulates alcohol-seeking.
Authors: Ryan Et al.
Proc Natl Acad Sci U S A 2013;110:20789
-
Icilin-evoked behavioral stimulation is attenuated by alpha2-adrenoceptor activation.
Authors: Kim Et al.
Brain Res 2011;1384:110
-
The genetic design of signaling cascades to record receptor activation.
Authors: Barnea Et al.
Proc Natl Acad Sci U S A 2008;105:64
-
Ectopic activity in the rat pulmonary vein can arise from simultaneous activation of alpha1- and beta1-adrenoceptors.
Authors: Maupoil Et al.
Br J Pharmacol 2007;150:899
-
Enhancing functional production of G protein-coupled receptors in Pichia pastoris to levels required for structural studies via a single expression screen.
Authors: André Et al.
Protein Sci 2006;15:1115
-
Haloperidol, but not clozapine, produces dramatic catalepsy in delta9-THC-treated rats: possible clinical implications.
Authors: Marchese Et al.
Br J Pharmacol 2003;140:520
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for Yohimbine hydrochloride
There are currently no reviews for this product. Be the first to review Yohimbine hydrochloride and earn rewards!
Have you used Yohimbine hydrochloride?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image