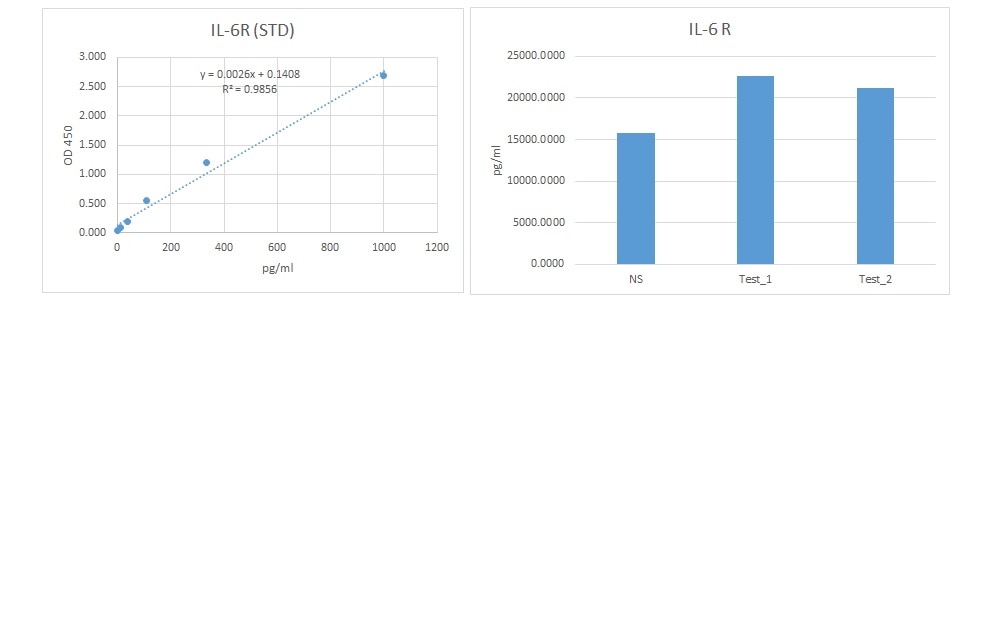
Human IL-6R alpha DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-6 sR. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
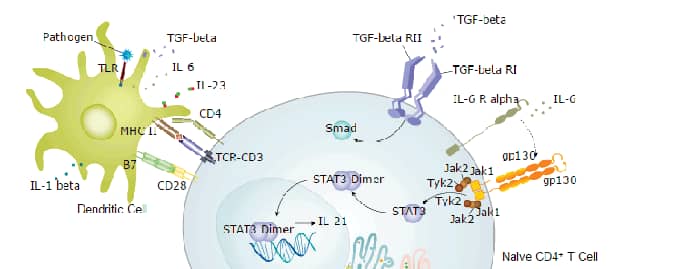
Background: IL-6R alpha
IL-6 R alpha is a transmembrane subunit of the receptor for Interleukin-6. It associates with gp130 which is a shared component of the receptors for CLC, CNTF, CT-1, IL-11, IL-27, LIF, and OSM. Soluble forms of IL-6 R alpha are generated by both alternative splicing and proteolytic cleavage. In a mechanism known as trans-signaling, complexes of soluble IL-6 and IL-6 R alpha elicit responses from gp130-expressing cells that lack cell surface IL-6 R alpha. IL-6 plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression. IL-6, along with TNF-alpha and IL-1, drives the acute inflammatory response and the transition from acute inflammation to either acquired immunity or chronic inflammatory disease. When dysregulated, it contributes to chronic inflammation in obesity, insulin resistance, inflammatory bowel disease, arthritis, sepsis, and atherosclerosis.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-6R alpha DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
18
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
SARS-CoV-2 infection results in immune responses in the respiratory tract and peripheral blood that suggest mechanisms of disease severity
Authors: W Zhang, BY Chua, KJ Selva, L Kedzierski, TM Ashhurst, ER Haycroft, SK Shoffner-B, L Hensen, DF Boyd, F James, E Mouhtouris, JC Kwong, KYL Chua, G Drewett, A Copaescu, JE Dobson, LC Rowntree, JR Habel, LF Allen, HF Koay, JA Neil, MJ Gartner, CY Lee, P Andersson, SF Khan, L Blakeway, J Wisniewski, JH McMahon, EE Vine, AL Cunningham, J Audsley, I Thevarajan, T Seemann, NL Sherry, F Amanat, F Krammer, SL Londrigan, LM Wakim, NJC King, DI Godfrey, LK Mackay, PG Thomas, S Nicholson, KB Arnold, AW Chung, NE Holmes, OC Smibert, JA Trubiano, CL Gordon, THO Nguyen, K Kedzierska
Nature Communications, 2022-05-19;13(1):2774.
Species: Human
Sample Types: Plasma
-
Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS
Authors: M Machci?ska, M Kierasi?sk, M Michniowsk, M Maruszewsk, L Szewczak, R Rola, A Karli?ska, M Stear, K Donskow-?y
International Journal of Molecular Sciences, 2022-03-16;23(6):.
Species: Human
Sample Types: Serum
-
Immune responses in COVID-19 respiratory tract and blood reveal mechanisms of disease severity
Authors: W Zhang, B Chua, K Selva, L Kedzierski, T Ashhurst, E Haycroft, S Shoffner, L Hensen, D Boyd, F James, E Mouhtouris, J Kwong, K Chua, G Drewett, A Copaescu, J Dobson, L Rowntree, J Habel, L Allen, HF Koay, J Neil, M Gartner, C Lee, P Andersson, T Seemann, N Sherry, F Amanat, F Krammer, S Londrigan, L Wakim, N King, D Godfrey, L Mackay, P Thomas, S Nicholson, K Arnold, A Chung, N Holmes, O Smibert, J Trubiano, C Gordon, T Nguyen, K Kedzierska
Research square, 2021-08-26;0(0):.
Species: Human
Sample Types: Plasma
-
Perturbation of the Actin Cytoskeleton in Human Hepatoma Cells Influences Interleukin-6 (IL-6) Signaling, but Not Soluble IL-6 Receptor Generation or NF-kappaB Activation
Authors: E Georgieva, SL Leber, C Wex, C Garbers
International Journal of Molecular Sciences, 2021-07-02;22(13):.
Species: Human
Sample Types: Cell Lysates
-
Interleukin-6 controls recycling and degradation, but not internalization of its receptors
Authors: CM Flynn, B Kespohl, T Daunke, Y Garbers, S Düsterhöft, S Rose-John, J Haybaeck, J Lokau, S Aparicio-S, C Garbers
The Journal of Biological Chemistry, 2021-02-19;0(0):100434.
Species: Human
Sample Types: Cell Culture Supernates
-
Cathepsin S provokes interleukin-6 (IL-6) trans-signaling through cleavage of the IL-6 receptor in vitro
Authors: CM Flynn, Y Garbers, S Düsterhöft, R Wichert, J Lokau, CHK Lehmann, D Dudziak, B Schröder, C Becker-Pau, S Rose-John, S Aparicio-S, C Garbers
Scientific Reports, 2020-12-10;10(1):21612.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency
Authors: M Werner-Kle, A Grujovic, C Irlbeck, M Obradovi?, M Hoffmann, H Koerkel-Qu, X Lu, S Treitschke, C Köstler, C Botteron, K Weidele, C Werno, B Polzer, S Kirsch, M Gužvi?, J Warfsmann, K Honarnejad, Z Czyz, G Feliciello, I Blochberge, S Grunewald, E Schneider, G Haunschild, N Patwary, S Guetter, S Huber, B Rack, N Harbeck, S Buchholz, P Rümmele, N Heine, S Rose-John, CA Klein
Nat Commun, 2020-10-05;11(1):4977.
Species: Human
Sample Types: Cell Culture Supernates
-
Trans IL-6 signaling does not appear to play a role in renal scarring after urinary tract infection
Authors: S Gupta, GY Junquera, L Nicassio, B Becknell, CB Ching
J Pediatr Urol, 2020-05-29;0(0):.
Species: Human
Sample Types: Urine
-
Activation of Toll-like Receptor 2 (TLR2) induces Interleukin-6 trans-signaling
Authors: CM Flynn, Y Garbers, J Lokau, D Wesch, DM Schulte, M Laudes, W Lieb, S Aparicio-S, C Garbers
Sci Rep, 2019-05-13;9(1):7306.
Species: Human
Sample Types: Serum
-
Therapeutic blockade of the interleukin-6 receptor (IL-6R) allows sIL-6R generation by proteolytic cleavage
Authors: N Prenissl, J Lokau, S Rose-John, J Haybaeck, C Garbers
Cytokine, 2018-12-14;114(0):1-5.
Species: Human, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
p63 is a key regulator of iRHOM2 signalling in the keratinocyte stress response
Authors: P Arcidiacon, CM Webb, MA Brooke, H Zhou, PJ Delaney, KE Ng, DC Blaydon, A Tinker, DP Kelsell, A Chikh
Nat Commun, 2018-03-09;9(1):1021.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women.
Authors: Dogan, Meeshant, Shields, Bridget, Cutrona, Carolyn, Gao, Long, Gibbons, Frederic, Simons, Ronald, Monick, Martha, Brody, Gene H, Tan, Kai, Beach, Steven R, Philibert, Robert A
BMC Genomics, 2014-02-22;15(0):151.
Species: Human
Sample Types: Serum
-
Anti-Tumour Effects of a Specific Anti-ADAM17 Antibody in an Ovarian Cancer Model In Vivo.
Authors: Richards FM, Tape CJ, Jodrell DI, Murphy G
PLoS ONE, 2012-07-11;7(7):e40597.
Species: Human
Sample Types: Tissue Homogenates
-
PI3K/p110{delta} is a novel therapeutic target in multiple myeloma.
Authors: Ikeda H, Hideshima T, Fulciniti M
Blood, 2010-05-26;116(9):1460-8.
Species: Human
Sample Types: Serum
-
Altered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with pre-eclampsia.
Authors: Zhao S, Gu Y, Dong Q, Fan R, Wang Y
Placenta, 2008-11-05;29(12):1024-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production.
Authors: Ahmed S, Marotte H, Kwan K, Ruth JH, Campbell PL, Rabquer BJ, Pakozdi A, Koch AE
Proc. Natl. Acad. Sci. U.S.A., 2008-09-16;105(38):14692-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells.
Authors: Briso EM, Dienz O, Rincon M
J. Immunol., 2008-06-01;180(11):7102-6.
Species: Human
Sample Types: Cell Culture Supernates
-
p38 mitogen-activated protein kinase inhibitor LY2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications.
Authors: Ishitsuka K, Hideshima T, Neri P, Vallet S, Shiraishi N, Okawa Y, Shen Z, Raje N, Kiziltepe T, Ocio EM, Chauhan D, Tassone P, Munshi N, Campbell RM, Dios AD, Shih C, Starling JJ, Tamura K, Anderson KC
Br. J. Haematol., 2008-04-07;141(5):598-606.
Species: Mouse
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-6R alpha DuoSet ELISA
Average Rating: 5 (Based on 2 Reviews)
Have you used Human IL-6R alpha DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: