Mouse CCL5/RANTES DuoSet ELISA
Mouse CCL5/RANTES DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse CCL5/RANTES. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL5/RANTES
RANTES (Regulated upon Activation, Normal T cell-expressed, and presumably Secreted), also known as CCL5, a member of the CC chemokine family of inflammatory and immunoregulatory cytokines, initially discovered by subtractive hybridization as a T cell-specific molecule (1, 2). Mouse RANTES cDNA encodes a 91 amino acid (aa) residue precursor protein with a presumed signal peptide of 23 aa residues that is cleaved to generate the 68 aa residue mature protein (3). At the amino acid sequence level, mouse RANTES is 84% identical to human RANTES. Both human and mouse RANTES exhibit cross species activity. Naturally occurring human RANTES has been found to be a mixture of the 68 aa residue mature protein and a 66 aa residue amino-terminally truncated form (4, 5). Cells known to express RANTES include keratinocytes, eosinophils, platelets, endothelium, fibroblasts, respiratory epithelium, astrocytes, vascular smooth muscle, and CD4+ and CD8+ T cells (6-14). As suggested by its acronym, RANTES production by the various cell types is induced in response to cytokine stimulation (8-13).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse CCL5/RANTES DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
78
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Induced pluripotent stem cell-derived extracellular vesicles promote wound repair in a diabetic mouse model via an anti-inflammatory immunomodulatory mechanism
Authors: D Levy, SN Abadchi, N Shababi, MR Ravari, NH Pirolli, C Bergeron, A Obiorah, F Mokhtari-E, S Gheshlaghi, JM Abraham, IM Smith, E Powsner, T Solomon, JW Harmon, SM Jay
bioRxiv : the preprint server for biology, 2023-03-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MYC promotes immune-suppression in triple-negative breast cancer via inhibition of interferon signaling
Authors: D Zimmerli, CS Brambillas, F Talens, J Bhin, R Linstra, L Romanens, A Bhattachar, SEP Joosten, AM Da Silva, N Padrao, MD Wellenstei, K Kersten, M de Boo, M Roorda, L Henneman, R de Bruijn, S Annunziato, E van der Bu, AP Drenth, C Lutz, T Endres, M van de Ven, M Eilers, L Wessels, KE de Visser, W Zwart, RSN Fehrmann, MATM van Vugt, J Jonkers
Nature Communications, 2022-11-02;13(1):6579.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protective and vaccine dose-sparing efficacy of Poly I:C-functionalized calcium phosphate nanoparticle adjuvants in inactivated influenza vaccination
Authors: J Lee, SY Ahn, CTT Le, DH Lee, J Jung, EJ Ko
International immunopharmacology, 2022-09-14;112(0):109240.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The IRAK4 scaffold integrates TLR4-driven TRIF and MYD88 signaling pathways
Authors: M Pereira, DF Durso, CE Bryant, EA Kurt-Jones, N Silverman, DT Golenbock, RT Gazzinelli
Cell Reports, 2022-08-16;40(7):111225.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron-Carbohydrate Nanoparticles from Serum
Authors: T Arsiwala, AS Vogt, AE Barton, V Manolova, F Funk, B Flühmann, MF Bachmann
International Journal of Molecular Sciences, 2022-02-28;23(5):.
Species: Mouse
Sample Types: Tissue Lysates
-
Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr-/-.Leiden Mice
Authors: MC Morrison, E Gart, WV Duyvenvoor, J Snabel, MJ Nielsen, DJ Leeming, A Menke, R Kleemann
International Journal of Molecular Sciences, 2022-02-19;23(4):.
Species: Mouse
Sample Types: Tissue Homogenates
-
CCL5 via GPX1 activation protects hippocampal memory function after mild traumatic brain injury
Authors: MH Ho, CH Yen, TH Hsieh, TJ Kao, JY Chiu, YH Chiang, BJ Hoffer, WC Chang, SY Chou
Redox Biology, 2021-07-17;46(0):102067.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ligase Pellino3 Regulates Macrophage Action and Survival in Response to VSV Infection in RIG-I-Dependent Path
Authors: P Reniewicz, A Kula, E Makuch, M Ochnik, T Lipi?ski, J Siednienko
Oxidative Medicine and Cellular Longevity, 2021-07-01;2021(0):6668463.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice
Authors: MTP de Oliveir, DS Coutinho, SS Guterres, AR Pohlmann, PMRE Silva, MA Martins, A Bernardi
Pharmaceutics, 2021-05-10;13(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Combination of subtherapeutic anti-TNF dose with dasatinib restores clinical and molecular arthritogenic profiles better than standard anti-TNF treatment
Authors: L Ntari, C Nikolaou, K Kranidioti, D Papadopoul, E Christodou, P Chouvardas, F Meier, C Geka, MC Denis, N Karagianni, G Kollias
Journal of Translational Medicine, 2021-04-23;19(1):165.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Respiratory Epithelial Cells Respond to Lactobacillus plantarum but Provide No Cross-Protection against Virus-Induced Inflammation
Authors: E Mai, CM Percopo, AR Limkar, AC Sek, M Ma, HF Rosenberg
Viruses, 2020-12-22;13(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leptospiral LPS escapes mouse TLR4 internalization and TRIF?associated antimicrobial responses through O antigen and associated lipoproteins
Authors: D Bonhomme, I Santecchia, F Vernel-Pau, M Caroff, P Germon, G Murray, B Adler, IG Boneca, C Werts
PLoS Pathog., 2020-08-13;16(8):e1008639.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy
Authors: MZ Noman, S Parpal, K Van Moer, M Xiao, Y Yu, J Viklund, A De Milito, M Hasmim, M Andersson, RK Amaravadi, J Martinsson, G Berchem, B Janji
Sci Adv, 2020-04-29;6(18):eaax7881.
Species: Mouse
Sample Types: Tissue Homogenates
-
Pre-treatment with the viral Toll-like receptor 3 agonist poly(I:C) modulates innate immunity and protects neutropenic mice infected intracerebrally with Escherichia coli
Authors: S Ribes, C Arcilla, M Ott, S Schütze, UK Hanisch, S Nessler, R Nau
J Neuroinflammation, 2020-01-17;17(1):24.
Species: Mouse
Sample Types: Tissue homoge
-
The alternative cap-binding complex is required for antiviral defense in vivo
Authors: A Gebhardt, V Bergant, D Schnepf, M Moser, A Meiler, D Togbe, C Mackowiak, LS Reinert, SR Paludan, B Ryffel, A Stukalov, P Staeheli, A Pichlmair
PLoS Pathog., 2019-12-19;15(12):e1008155.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PTP1B negatively regulates nitric oxide-mediated Pseudomonas aeruginosa killing by neutrophils
Authors: L Yue, M Yan, ML Tremblay, TJ Lin, H Li, T Yang, X Song, T Xie, Z Xie
PLoS ONE, 2019-09-18;14(9):e0222753.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
Innate immune memory through TLR2 and NOD2 contributes to the control of Leptospira interrogans infection
Authors: I Santecchia, F Vernel-Pau, O Rasid, J Quintin, M Gomes-Sole, IG Boneca, C Werts
PLoS Pathog., 2019-05-20;15(5):e1007811.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Translational repression of Ccl5 and Cxcl10 by 4E-BP1 and 4E-BP2 restrains the ability of mouse macrophages to induce migration of activated T�cells
Authors: M William, LP Leroux, V Chaparro, TE Graber, T Alain, M Jaramillo
Eur. J. Immunol., 2019-05-06;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of a fluid-phase PRR in fighting an intracellular pathogen: PTX3 in Shigella infection
Authors: V Ciancarell, L Lembo-Fazi, I Paciello, AK Bruno, S Jaillon, S Berardi, M Barbagallo, S Meron-Suda, D Cohen, A Molinaro, G Rossi, C Garlanda, ML Bernardini
PLoS Pathog., 2018-12-07;14(12):e1007469.
Species: Mouse
Sample Types: Tissue Homogenates
-
Microphthalmia-Associated Transcription Factor (MITF) Regulates Immune Cell Migration into Melanoma
Authors: GM Wiedemann, C Aithal, A Kraechan, C Heise, BL Cadilha, J Zhang, P Duewell, R Ballotti, S Endres, C Bertolotto, S Kobold
Transl Oncol, 2018-11-28;12(2):350-360.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MiR-188-3p upregulation results in the inhibition of macrophage proinflammatory activities and atherosclerosis in ApoE-deficient mice
Authors: XF Zhang, Y Yang, XY Yang, Q Tong
Thromb. Res., 2018-09-05;171(0):55-61.
Species: Mouse
Sample Types: Plasma
-
Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations
Authors: A Singanayag, N Glanville, JL Girkin, YM Ching, A Marcellini, JD Porter, M Toussaint, RP Walton, LJ Finney, J Aniscenko, J Zhu, MB Trujillo-T, MA Calderazzo, C Grainge, SL Loo, PC Veerati, PS Pathinayak, KS Nichol, AT Reid, PL James, R Solari, PAB Wark, DA Knight, MF Moffatt, WO Cookson, MR Edwards, P Mallia, NW Bartlett, SL Johnston
Nat Commun, 2018-06-08;9(1):2229.
Species: Mouse
Sample Types: BALF
-
Comparing Flow Cytometry QBeads PlexScreen Assays with Other Immunoassays for Determining Multiple Analytes
Authors: M Ding, A Cavallin, NO Hermansson, P Berntsson, L Jinton, S Rodrigo Bl
SLAS Discov, 2018-04-24;0(0):2472555218771.
Species: Mouse
Sample Types: BALF
-
Genetic Polymorphism at CCL5 Is Associated With Protection in Chagas' Heart Disease: Antagonistic Participation of CCR1+ and CCR5+ Cells in Chronic Chagasic Cardiomyopathy.
Authors: Angelica Martins Batista, Lucia Elena Alvarado-, Silvia Marinho Alves, Gloria Melo, Isabela Resende Pereira, Leonardo Alexandre de Sou Ruivo, Andrea Alice Da Silva, Daniel Gibaldi, Thayse Do E S Protásio Da Silva, Virginia Maria Barros De Lorena, Adriene Siqueira De Melo, Ana Karine De Araújo, Michelle Da Silva Barros, Vláudia Maria Assis Costa, Cynthia C Cardoso, Antonio G Pacheco, Cristina Carrazzon, Wilson Oliveira, Milton Ozório Moraes, Joseli Lannes-Vi
Frontiers in Immunology, 2018-04-11;0(0):1664-3224.
Species: Mouse
Sample Types: Tissue Homogenates
-
Chronic Intake of the Selective Serotonin Reuptake Inhibitor Fluoxetine Enhances Atherosclerosis
Authors: M Rami, R Guillamat-, P Rinne, M Salvermose, L Ring, M Bianchini, X Blanchet, RTA Megens, Y Döring, B Walzog, O Soehnlein, C Weber, A Faussner, S Steffens
Arterioscler. Thromb. Vasc. Biol., 2018-03-22;0(0):.
Species: Mouse
Sample Types: Serum
-
Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation
Authors: D Fang, K Cui, G Hu, RK Gurram, C Zhong, AJ Oler, R Yagi, M Zhao, S Sharma, P Liu, B Sun, K Zhao, J Zhu
J. Exp. Med., 2018-03-07;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nlrp12 Mediates Adverse Neutrophil Recruitment during Influenza Virus Infection
Authors: EE Hornick, B Banoth, AM Miller, ZR Zacharias, N Jain, ME Wilson, KN Gibson-Cor, KL Legge, GA Bishop, FS Sutterwala, SL Cassel
J. Immunol., 2017-12-27;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Neuronal ICAM-5 Inhibits Microglia Adhesion and Phagocytosis and Promotes an Anti-inflammatory Response in LPS Stimulated Microglia
Authors: S Paetau, T Rolova, L Ning, CG Gahmberg
Front Mol Neurosci, 2017-12-22;10(0):431.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cathelicidins Inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner
Authors: M Coorens, VAF Schneider, AM de Groot, A van Dijk, M Meijerink, JM Wells, MR Scheenstra, EJA Veldhuizen, HP Haagsman
J. Immunol., 2017-07-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Deep-Sea Polyextremophile Halobacteroides lacunaris TB21 Rough-Type LPS: Structure and Inhibitory Activity towards Toxic LPS
Authors: FD Lorenzo, A Palmigiano, I Paciello, M Pallach, D Garozzo, ML Bernardini, V Cono, MM Yakimov, A Molinaro, A Silipo
Mar Drugs, 2017-06-27;15(7):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin 17A Promotes Lymphocytes Adhesion and Induces CCL2 and CXCL1 Release from Brain Endothelial Cells
Authors: DW Wojkowska, P Szpakowski, A Glabinski
Int J Mol Sci, 2017-05-08;18(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protein malnutrition promotes dysregulation of molecules involved in T cell migration in the thymus of mice infected with Leishmania infantum
Authors: M Losada-Bar, A Umaña-Pére, S Cuervo-Esc, LR Berbert, R Porrozzi, FN Morgado, DA Mendes-da-, W Savino, M Sánchez-Gó, P Cuervo
Sci Rep, 2017-04-11;7(0):45991.
Species: Mouse
Sample Types: Interstitial Fluid
-
Contributions of CD8 T cells to the pathogenesis of mouse adenovirus type 1 respiratory infection
Authors: CT Molloy, JS Andonian, HM Seltzer, MC Procario, ME Watson, JB Weinberg
Virology, 2017-04-11;507(0):64-74.
Species: Mouse
Sample Types: BALF
-
Biglycan- and Sphingosine Kinase-1 Signaling Crosstalk Regulates the Synthesis of Macrophage Chemoattractants
Authors: LT Hsieh, MV Nastase, H Roedig, J Zeng-Brouw, C Poluzzi, S Schwalm, C Fork, C Tredup, RP Brandes, M Wygrecka, A Huwiler, J Pfeilschif, L Schaefer
Int J Mol Sci, 2017-03-09;18(3):.
Species: Human
Sample Types: Plasma
-
The protective effect of the anti-Toll-like receptor 9 antibody against acute cytokine storm caused by immunostimulatory DNA
Authors: Y Murakami, R Fukui, Y Motoi, T Shibata, SI Saitoh, R Sato, K Miyake
Sci Rep, 2017-03-07;7(0):44042.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Antibodies against nonstructural protein 1 protect mice from dengue virus-induced mast cell activation
Authors: YT Chu, SW Wan, YC Chang, CK Lee, BA Wu-Hsieh, R Anderson, YS Lin
Lab. Invest, 2017-02-27;0(0):.
Species: Mouse
Sample Types: Serum
-
The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2
Authors: T Hartwig, A Montinaro, S von Karste, A Sevko, S Surinova, A Chakravart, L Taraborrel, P Draber, E Lafont, F Arce Varga, MA El-Bahrawy, SA Quezada, H Walczak
Mol. Cell, 2017-02-16;65(4):730-742.e5.
Species: Mouse
Sample Types: Tissue Homogenates
-
Deletion of the Chemokine Binding Protein Gene from the Parapoxvirus Orf Virus Reduces Virulence and Pathogenesis in Sheep
Authors: SB Fleming, C McCaughan, Z Lateef, A Dunn, LM Wise, NC Real, AA Mercer
Front Microbiol, 2017-01-24;8(0):46.
Species: Mouse
Sample Types: Protein
-
Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis
Authors: M Coorens, MR Scheenstra, EJ Veldhuizen, HP Haagsman
Sci Rep, 2017-01-19;7(0):40874.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis
Authors: Andrey Lozhkin
J. Mol. Cell. Cardiol, 2016-12-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protease-Activated Receptor 1 Enhances Poly I:C Induction of the Antiviral Response in Macrophages and Mice
Authors: Nigel Mackman
J Innate Immun, 2016-11-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
P2X7 receptor-dependent tuning of gut epithelial responses to infection
Authors: SW Huang, C Walker, J Pennock, K Else, W Muller, MJ Daniels, C Pellegrini, D Brough, G Lopez-Cast, SM Cruickshan
Immunol. Cell Biol, 2016-08-25;95(2):178-188.
Species: Mouse
Sample Types: Serum
-
Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection.
Authors: Brown A, Murphy A, Lalor S, Leech J, O'Keeffe K, Mac Aogain M, O'Halloran D, Lacey K, Tavakol M, Hearnden C, Fitzgerald-Hughes D, Humphreys H, Fennell J, van Wamel W, Foster T, Geoghegan J, Lavelle E, Rogers T, McLoughlin R
PLoS Pathog, 2015-11-05;11(11):e1005226.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ethanolic Echinacea purpurea Extracts Contain a Mixture of Cytokine-Suppressive and Cytokine-Inducing Compounds, Including Some That Originate from Endophytic Bacteria.
Authors: Todd D, Gulledge T, Britton E, Oberhofer M, Leyte-Lugo M, Moody A, Shymanovich T, Grubbs L, Juzumaite M, Graf T, Oberlies N, Faeth S, Laster S, Cech N
PLoS ONE, 2015-05-01;10(5):e0124276.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases.
Authors: Sottnik J, Dai J, Zhang H, Campbell B, Keller E
Cancer Res, 2015-04-08;75(11):2151-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phosphatidylinositol 3-Kinase gamma is required for the development of experimental cerebral malaria.
Authors: Lacerda-Queiroz N, Brant F, Rodrigues D, Vago J, Rachid M, Sousa L, Teixeira M, Teixeira A
PLoS ONE, 2015-03-16;10(3):e0119633.
Species: Mouse
Sample Types: Serum
-
Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model.
Authors: Verheijden K, Willemsen L, Braber S, Leusink-Muis T, Delsing D, Garssen J, Kraneveld A, Folkerts G
Respir Res, 2015-02-07;16(0):17.
Species: Mouse
Sample Types: Tissue Homogenates
-
Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production.
Authors: Kearney C, Cullen S, Tynan G, Henry C, Clancy D, Lavelle E, Martin S
Cell Death Differ, 2015-01-23;22(8):1313-27.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-15 modulates adipose tissue by altering mitochondrial mass and activity.
Authors: Barra, Nicole G, Palanivel, Rengasam, Denou, Emmanuel, Chew, Marianne, Gillgrass, Amy, Walker, Tina D, Kong, Josh, Richards, Carl D, Jordana, Manel, Collins, Stephen, Trigatti, Bernardo, Holloway, Alison C, Raha, Sandeep, Steinberg, Gregory, Ashkar, Ali A
PLoS ONE, 2014-12-17;9(12):e114799.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Fli-1 transcription factor regulates the expression of CCL5/RANTES.
Authors: Lennard Richard M, Sato S, Suzuki E, Williams S, Nowling T, Zhang X
J Immunol, 2014-08-06;193(6):2661-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy.
Authors: Walsh K, Teijaro J, Brock L, Fremgen D, Collins P, Rosen H, Oldstone M
J Virol, 2014-03-26;88(11):6281-93.
Species: Mouse
Sample Types: BALF
-
Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection.
Authors: Teijaro J, Walsh K, Rice S, Rosen H, Oldstone M
Proc Natl Acad Sci U S A, 2014-02-26;111(10):3799-804.
Species: Mouse
Sample Types: BALF
-
Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion.
Authors: Menezes-Garcia Z, Oliveira M, Lima R, Soriani F, Cisalpino D, Botion L, Teixeira M, Souza D, Ferreira A
Obesity (Silver Spring), 2013-12-14;22(3):663-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12.
Authors: Paulissen G, El Hour M, Rocks N, Gueders M, Bureau F, Foidart J, Lopez-Otin C, Noel A, Cataldo D
J Immunol, 2012-09-07;189(8):4135-43.
Species: Mouse
Sample Types: Tissue Homogenates
-
Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, Is crucial for murine asthma.
Authors: Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, Yamaguchi S, Suzukawa K, Nakanishi W, Oboki K, Kajiwara N, Ohno T, Ishii A, Korner H, Cua D, Suto H, Yoshimoto T, Iwakura Y, Yamasoba T, Ohta K, Sudo K, Saito H, Okumura K, Broide D, Matsumoto K, Nakae S
J Immunol, 2012-08-31;189(7):3641-52.
Species: Mouse
Sample Types: BALF
-
Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo.
Authors: Singh PP, Smith VL, Karakousis PC, Schorey JS
J. Immunol., 2012-06-20;189(2):777-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems.
Authors: Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, Fernandez C
PLoS ONE, 2012-02-29;7(2):e32125.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification and functional characterization of novel phosphorylation sites in TAK1-binding protein (TAB) 1.
Authors: Wolf A, Beuerlein K, Eckart C, Weiser H, Dickkopf B, Muller H, Sakurai H, Kracht M
PLoS ONE, 2011-12-22;6(12):e29256.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection.
Authors: Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H
Cell, 2011-09-16;146(6):980-91.
Species: Mouse
Sample Types: BALF
-
Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus.
Authors: Walsh KB, Teijaro JR, Wilker PR
Proc. Natl. Acad. Sci. U.S.A., 2011-06-29;108(29):12018-23.
Species: Mouse
Sample Types: BALF
-
Chemokine binding protein M3 of murine gammaherpesvirus 68 modulates the host response to infection in a natural host.
Authors: Hughes DJ, Kipar A, Leeming GH, Bennett E, Howarth D, Cummerson JA, Papoula-Pereira R, Flanagan BF, Sample JT, Stewart JP
PLoS Pathog., 2011-03-17;7(3):e1001321.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid.
Authors: van Neerven S, Regen T, Wolf D, Nemes A, Johann S, Beyer C, Hanisch UK, Mey J
J. Neurochem., 2010-06-16;114(5):1511-26.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chorionic gonadotropin alleviates thioglycollate-induced peritonitis by affecting macrophage function.
Authors: Wan H, Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Van Rooijen N, Khan NA, Kiekens RC, Benner R, Versnel MA
J. Leukoc. Biol., 2009-05-04;86(2):361-70.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
IFN-gamma synergizes with LPS to induce nitric oxide biosynthesis through glycogen synthase kinase-3-inhibited IL-10.
Authors: Lin CF, Tsai CC, Huang WC, Wang CY, Tseng HC, Wang Y, Kai JI, Wang SW, Cheng YL
J. Cell. Biochem., 2008-10-15;105(3):746-55.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes.
Authors: Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW, Chern Y
J. Neurosci., 2008-03-26;28(13):3277-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection.
Authors: Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC
J. Immunol., 2008-01-15;180(2):1080-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes.
Authors: Arias MA, Jaramillo G, Lopez YP, Mejia N, Mejia C, Pantoja AE, Shattock RJ, Garcia LF, Griffin GE
J. Immunol., 2007-12-15;179(12):8381-91.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential ex vivo nitric oxide production by acutely isolated neonatal and adult microglia.
Authors: Schell JB, Crane CA, Smith MF, Roberts MR
J. Neuroimmunol., 2007-08-15;189(1):75-87.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism.
Authors: Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF
FASEB J., 2007-07-11;21(14):3877-84.
Species: Mouse
Sample Types: Tissue Homogenates
-
Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis.
Authors: Hegde A, Zhang H, Moochhala SM, Bhatia M
J. Leukoc. Biol., 2007-06-12;82(3):678-85.
Species: Mouse
Sample Types: Plasma
-
Interleukin-17 is a negative regulator of established allergic asthma.
Authors: Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B
J. Exp. Med., 2006-11-13;203(12):2715-25.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Suppressive effect of simvastatin on interferon-beta-induced expression of CC chemokine ligand 5 in microglia.
Authors: Nakamichi K, Saiki M, Kitani H, Kuboyama Y, Morimoto K, Takayama-Ito M, Kurane I
Neurosci. Lett., 2006-09-15;407(3):205-10.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes.
Authors: Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM
J. Virol., 2006-06-01;80(11):5283-91.
Species: Mouse
Sample Types: Tissue Homogenates
-
MIP-1 gamma promotes receptor-activator-of-NF-kappa-B-ligand-induced osteoclast formation and survival.
Authors: Okamatsu Y, Battaglino R, Spate U, Stashenko P
J. Immunol., 2004-08-01;173(3):2084-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Specific engagement of TLR4 or TLR3 does not lead to IFN-beta-mediated innate signal amplification and STAT1 phosphorylation in resident murine alveolar macrophages.
Authors: Punturieri A, Alviani RS, Polak T, Copper P, Sonstein J, Curtis JL
J. Immunol., 2004-07-15;173(2):1033-42.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Orally administered CpG oligodeoxynucleotide induces production of CXC and CC chemokines in the gastric mucosa and suppresses bacterial colonization in a mouse model of Helicobacter pylori infection.
Authors: Raghavan S, Nystrom J, Fredriksson M, Holmgren J, Harandi AM
Infect. Immun., 2003-12-01;71(12):7014-22.
Species: Mouse
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CCL5/RANTES DuoSet ELISA
Average Rating: 4.9 (Based on 11 Reviews)
Have you used Mouse CCL5/RANTES DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Mice (HIII and LIII strains) injected i.p. with 2,4,6,10 tetramethylpentadecane (pristane).
used for ELISA, worked perfectly.
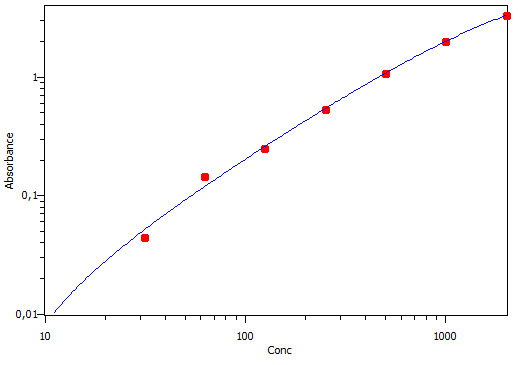
50ul of murine RANTES Capture antibody (2ug/mL) was coated per well of a 96 well plate at 4 degrees overnight. Purified RANTES standard was diluted (7 fold) and assayed to ensure the quality of the ELISA. Mouse RANTES Detector antibody was added (2h @ RT) followed by a 20 min incubation with Streptavidin-HRP. Substrate solution was added to measure RANTES concentration according to the manufactures protocol. The standard curve looked great as can be seen by calculation of the R- squared value. Highly recommend doing ELISA's using kits from R&D.
Buffer: 7-point standard curve