Recombinant Human FGFR1 alpha (IIIc) Fc Avi-tag Protein, CF
Recombinant Human FGFR1 alpha (IIIc) Fc Avi-tag Protein, CF Summary
Learn more about Avi-tag Biotinylated ProteinsProduct Specifications
| Human FGF R1 alpha (IIIc) (Arg22-Glu376) Accession # NP_075598.2 | IEGRDMD | Human IgG1 (Pro100-Lys330) | Avi-tag |
| N-terminus | C-terminus | ||
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
AVI11031
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
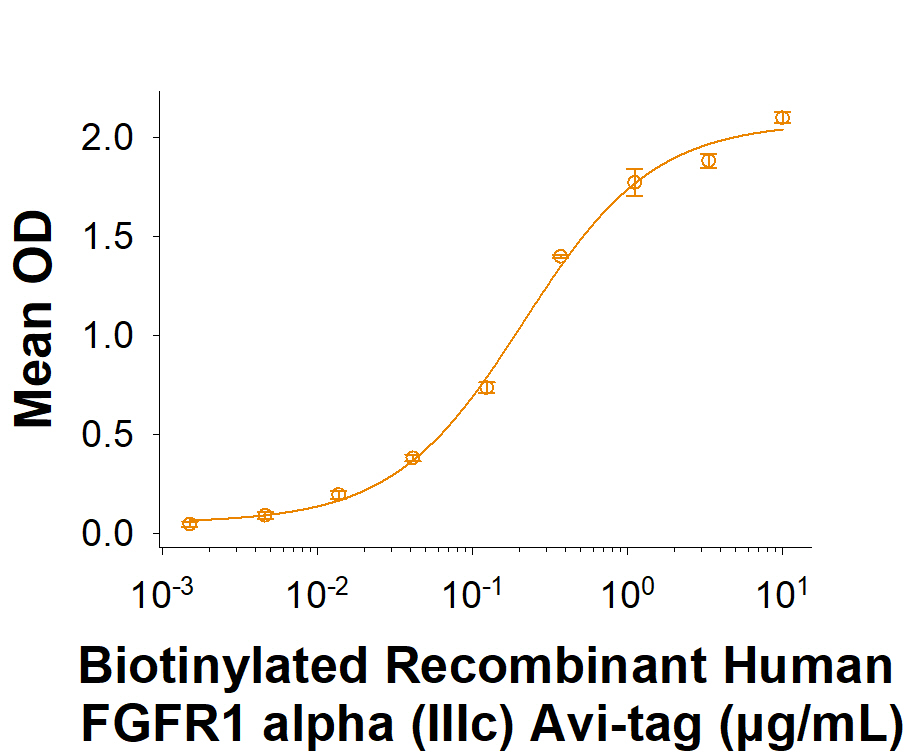 View Larger
View Larger
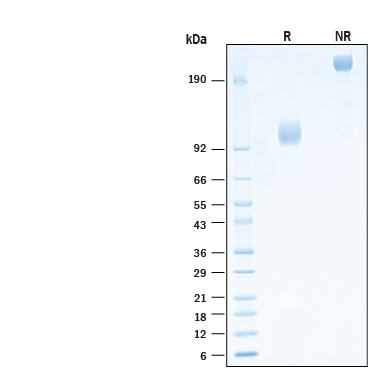 View Larger
View Larger
2 μg/lane of Biotinylated Recombinant Human FGFR1 alpha (IIIc) Fc Chimera Avi-tag Protein (Catalog # AVI11031) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 100-120 kDa and 200-240 kDa, respectively.
Reconstitution Calculator
Background: FGFR1 alpha
Fibroblast growth factor receptor 1 (FGFR1) belongs to a family of type I transmembrane tyrosine kinases which mediate the biological functions of FGFs that are involved in a multitude of physiological and pathological cellular processes (1). The FGFR family is comprised of 4 structurally conserved members (FGFR1-4) all possessing an extracellular domain (ECD) with three immunoglobulin (Ig)-like domains, an acid-box region containing a run of acidic residues between the IgI and IgII domains, a transmembrane domain and the split tyrosine-kinase domain (1, 2). The ECD of mature, full-length FGFR1 shares 98% amino acid sequence identity with mouse FGFR1. Alternative splicing generates multiple forms of FGFR1-3, each with unique signaling characteristics (3). For FGFR1, alternative splicing of the ECD generates FGFR1A, FGFR1B, and FGFR1G isoforms of the receptor with the A isoform containing three Ig domains, while the B and G isoforms lack the N-terminal IgI domain (3). Additional splicing of the IgIII domain, results in IIIa, IIIb, or IIIc isoforms (3). Only the alpha isoform has been identified for FGFR3 and FGFR4 and FGFR4 also lacks the IIIb and IIIc splicing events (4). The FGFR splice variants also exhibit distinct and varying binding affinities for different FGF ligands (2). FGFRs mediate the FGF signaling cascade which regulate developmental processes including cellular proliferation, differentiation, and migration, morphogenesis, and patterning (5). FGFRs transduce the signals through three dominant pathways including RAS/MAPK, PI3k/AKT, and PLC gamma (6). FGFR1 the most abundant FGFR and is widely expressed in many adult human tissues, but the splice variants display distinct tissue-specific differences with IIIc splice variants expressed in mesenchymal tissue (4, 7, 8). Mutations in FGFR1 or misregulation of FGFR1 mediated signaling is found in multiple diseases, with FGFR1A(IIIc) specifically upregulated, from breast and pancreatic cancer to Pfeiffer syndrome and osteoarthritis (4, 9-11). A soluble version of the FGFR1A(IIIc) splice variant has shown anti- angiogenic and anti-proliferative properties in multiple cancer cell line models (11). Our Avi-tag Biotinylated FGFR1A(IIIc) features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
- Ornitz, D.M. and Itoh, N. (2015) Wiley Interdiscip Rev Dev Biol. 4:215.
- Zhang, X. et al. (2006) J Biol Chem. 281:15694.
- Ferguson, H.R. et al. (2021) Signaling. Cells 10:1201.
- Holzmann, K. et al. (2012) J. Nucleic. Acids. 2012:950508.
- Xie, Y. et al. (2020) Sig Transduct Target Ther. 5:181.
- Mossahebi-Mohammadi, M. et al. (2020) Front Cell Dev. Biol. 18:79.
- Hughes, S.E. (1997) J. Histochem Cytochem. 45:1005.
- Delezoide, A.L. et al. (1998) Mech Dev. 77:19.
- Yamashita-Sugahara, Y. et al. (2016) Sci Rep 6:35908.
- Teven, C.M. et al. (2014) Genes Dis. 1:199.
- Babina, I. and Turner, N. (2017) Nat. Rev. Cancer 17:318.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human FGFR1 alpha (IIIc) Fc Avi-tag Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human FGFR1 alpha (IIIc) Fc Avi-tag Protein, CF and earn rewards!
Have you used Recombinant Human FGFR1 alpha (IIIc) Fc Avi-tag Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥1250 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image




