Human CCR7 Fluorescein-conjugated Antibody
Human CCR7 Fluorescein-conjugated Antibody Summary
Met1-Pro378
Accession # AAA58615
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
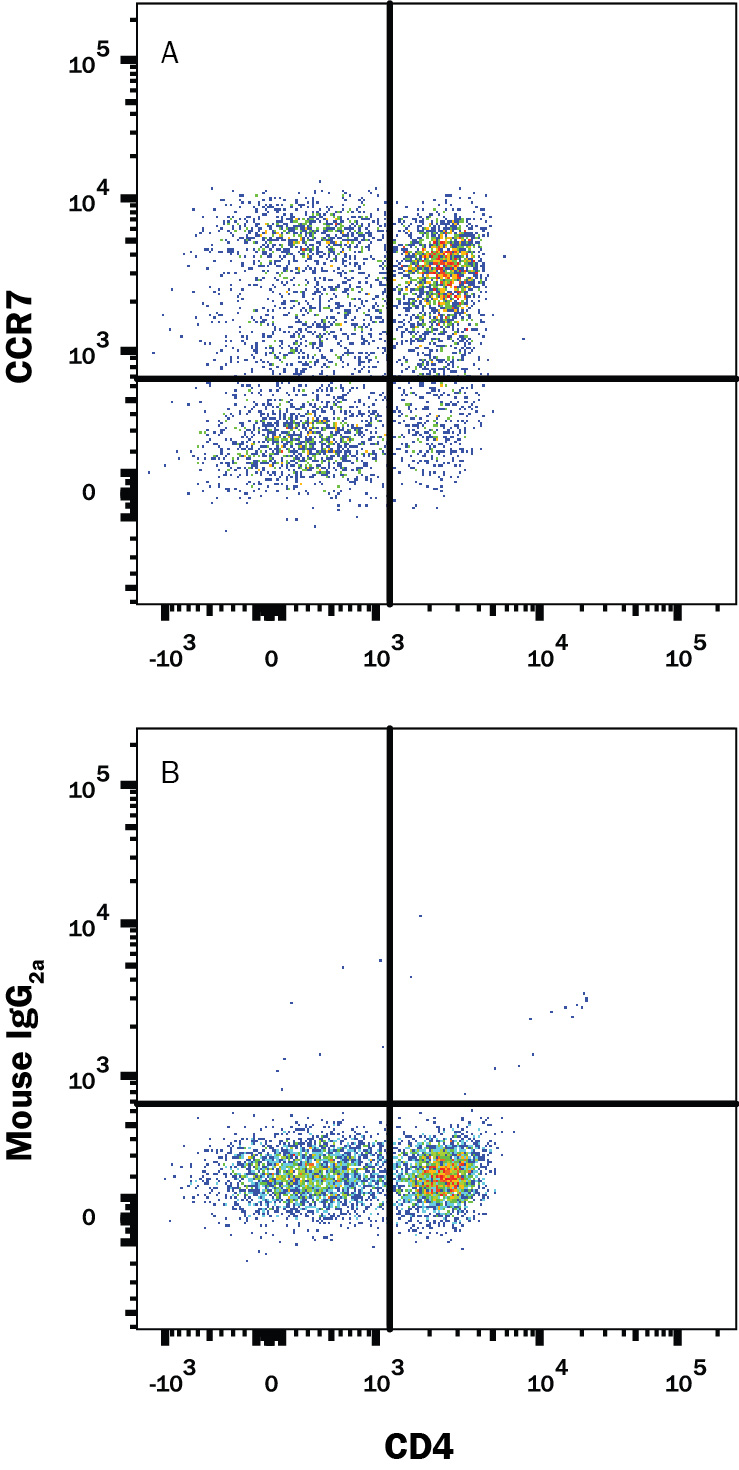 View Larger
View Larger
Detection of CCR7 in Human Blood Lymphocytes by Flow Cytometry. Human peripheral blood lymphocytes were stained with Mouse Anti-Human CD4 APC-conjugated Monoclonal Antibody (Catalog # FAB3791A) and either (A) Mouse Anti-Human CCR7 Fluorescein-conjugated Monoclonal Antibody (Catalog # FAB197F) or (B) Mouse IgG2AFluorescein Isotype Control (Catalog # IC003F). View our protocol for Staining Membrane-associated Proteins.
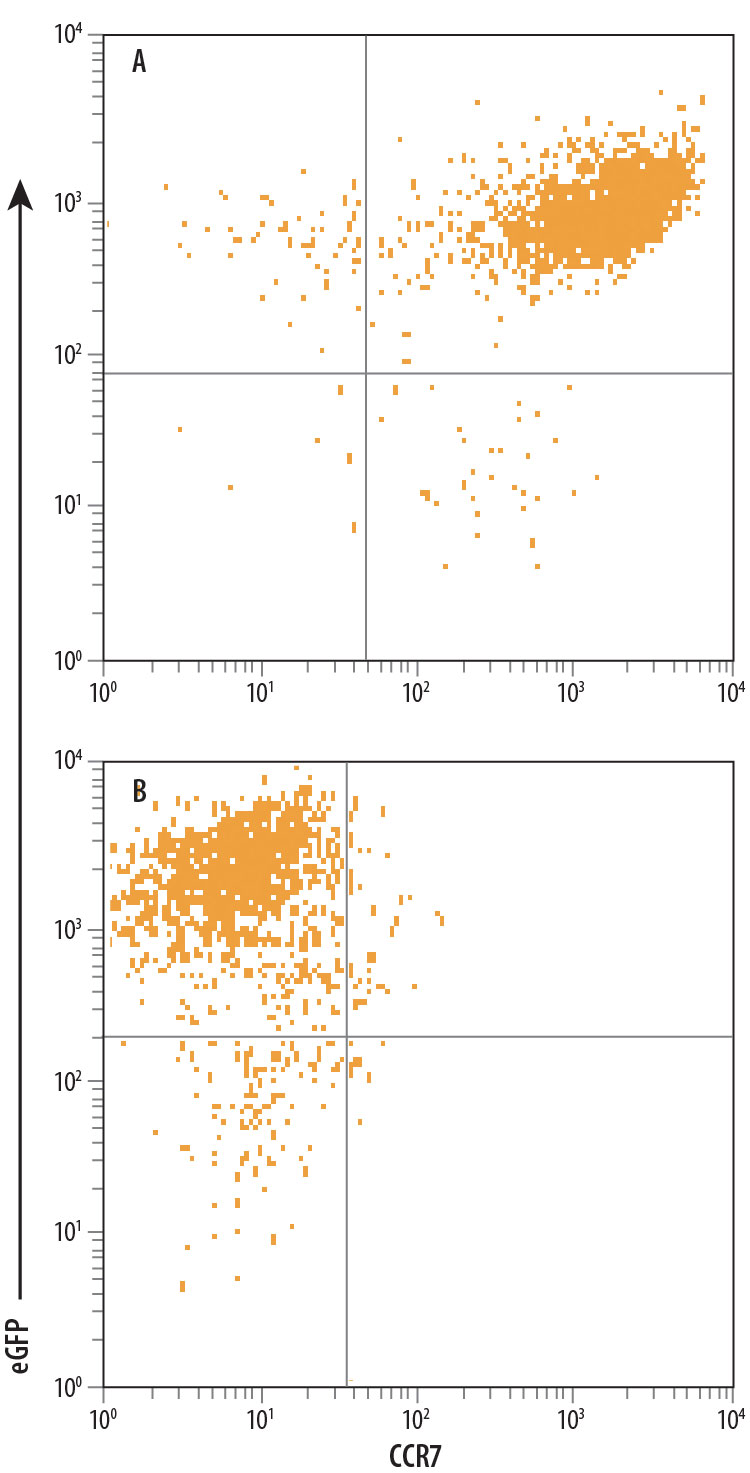 View Larger
View Larger
Detection of CCR7 in HEK293 Human Cell Line Transfected with Human CCR7 and eGFP by Flow Cytometry. The specificity of Mouse Anti-Human CCR7 Clone 150503 was demonstrated by its ability to react with (A) HEK293 human embryonic kidney cell line transfected with human CCR7 and not react with (B) irrelevant HEK293 transfectants. Antibody binding was monitored using Phycoerythrin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0102B).
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: CCR7
CCR7 (Chemokine Receptor 7; also CD197) is a 7 transmembrane (7TM) G protein-coupled chemokine receptor for the homeostatic chemokines CCL19/MIP-3 beta and CCL21/6Ckine. CCL19 and CCL21 are constitutively expressed by high endothelial venule epithelial cells or fibroblastic reticular cells in secondary lymphoid organs. CCR7 is upregulated on dendritic cells, naïve and memory T cells, Treg, NK cells, and B cells following inflammatory stimulation. Its expression enables the function of immune cell trafficking to and retention in regional lymph nodes for expansion of the adaptive immune response. Human CCR7 shares 87% amino acid sequence identity with mouse CCR7.
Product Datasheets
Citations for Human CCR7 Fluorescein-conjugated Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
67
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Reconstitution of EBV-directed T cell immunity by adoptive transfer of peptide-stimulated T cells in a patient after allogeneic stem cell transplantation for AITL
Authors: MF Lammoglia, J Ritter, R Gary, V Seitz, J Mautner, M Aigner, S Völkl, S Schaffer, S Moi, A Seegebarth, H Bruns, W Rösler, K Amann, M Büttner-He, S Hennig, A Mackensen, M Hummel, A Moosmann, A Gerbitz
PloS Pathogens, 2022-04-22;18(4):e1010206.
Species: Human
Sample Types:
Applications: Flow Cytometry -
Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells
Authors: P Agarwalla, EA Ogunnaike, S Ahn, KA Froehlich, A Jansson, FS Ligler, G Dotti, Y Brudno
Nature Biotechnology, 2022-03-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
BRAF and MEK Inhibitors Affect Dendritic-Cell Maturation and T-Cell Stimulation
Authors: S Hoyer, V Eberlein, G Schuler, C Berking, L Heinzerlin, N Schaft, J Dörrie
Oncogene, 2021-11-04;22(21):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
PD-1 is imprinted on cytomegalovirus-specific CD4+ T cells and attenuates Th1 cytokine production whilst maintaining cytotoxicity
Authors: HM Parry, AC Dowell, J Zuo, K Verma, FAM Kinsella, J Begum, W Croft, A Sharma-Oat, G Pratt, P Moss
PloS Pathogens, 2021-03-04;17(3):e1009349.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SARS-CoV-2 induces human plasmacytoid pre-dendritic cell diversification via UNC93B and IRAK4
Authors: F Onodi, L Bonnet-Mad, L Meertens, L Karpf, J Poirot, SY Zhang, C Picard, A Puel, E Jouanguy, Q Zhang, J Le Goff, JM Molina, C Delaugerre, JL Casanova, A Amara, V Soumelis
bioRxiv : the preprint server for biology, 2021-01-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
IL-18 Responsiveness Defines Limitations in Immune Help for Specialized FcR&gamma- NK Cells
Authors: RR Anderko, CR Rinaldo, RB Mailliard
J Immunol, 2020-11-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Insight into the mechanism of cytotoxicity of membrane-permeant psoralenic Kv1.3 channel inhibitors by chemical dissection of a novel member of the family
Authors: R Peruzzo, A Mattarei, M Azzolini, KA Becker-Fle, M Romio, G Rigoni, A Carrer, L Biasutto, S Parrasia, S Kadow, A Managò, A Urbani, A Rossa, G Semenzato, ME Soriano, L Trentin, S Ahmad, M Edwards, E Gulbins, C Paradisi, M Zoratti, L Leanza, I Szabò
Redox Biol, 2020-09-06;37(0):101705.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Chemokine Receptor CCR7 Triggers an Endomembrane Signaling Complex for Spatial Rac Activation
Authors: JM Laufer, MA Hauser, I Kindinger, V Purvanov, A Pauli, DF Legler
Cell Rep, 2019-10-22;29(4):995-1009.e6.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
TCR activation mimics CD127lowPD-1high phenotype and functional alterations of T lymphocytes from septic shock patients
Authors: J Mouillaux, C Allam, M Gossez, T Uberti, B Delwarde, J Hayman, T Rimmelé, J Textoris, G Monneret, E Peronnet, F Venet
Crit Care, 2019-04-17;23(1):131.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cancer immunotherapy with T cells carrying bispecific receptors that mimic antibodies
Authors: S Ahn, J Li, C Sun, K Gao, K Hirabayash, H Li, B Savoldo, R Liu, G Dotti
Cancer Immunol Res, 2019-03-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: CAR-T (Flow Cytometry) -
CD226 opposes TIGIT to disrupt Tregs in melanoma
Authors: J Fourcade, Z Sun, JM Chauvin, M Ka, D Davar, O Pagliano, H Wang, S Saada, C Menna, R Amin, C Sander, JM Kirkwood, AJ Korman, HM Zarour
JCI Insight, 2018-07-26;3(14):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Innate and adaptive immune dysregulation in critically ill ICU patients
Authors: NA Duggal, C Snelson, U Shaheen, V Pearce, JM Lord
Sci Rep, 2018-07-05;8(1):10186.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules
Authors: A Wagner, E Garner-Spi, J Jasinska, H Kollaritsc, K Stiasny, M Kundi, U Wiedermann
Sci Rep, 2018-06-29;8(1):9825.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Clinical-grade generation of peptide-stimulated CMV/EBV-specific T cells from G-CSF mobilized stem cell grafts
Authors: R Gary, M Aigner, S Moi, S Schaffer, A Gottmann, S Maas, R Zimmermann, J Zingsem, J Strobel, A Mackensen, J Mautner, A Moosmann, A Gerbitz
J Transl Med, 2018-05-09;16(1):124.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
PD-1 and PD-L1 Up-regulation Promotes T-cell Apoptosis in Gastric Adenocarcinoma
Authors: YM Chiu, CL Tsai, JT Kao, CT Hsieh, DC Shieh, YJ Lee, GJ Tsay, KS Cheng, YY Wu
Anticancer Res., 2018-04-01;38(4):2069-2078.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors
Authors: C Gavegnano, JH Brehm, FP Dupuy, A Talla, SP Ribeiro, DA Kulpa, C Cameron, S Santos, SJ Hurwitz, VC Marconi, JP Routy, L Sabbagh, RF Schinazi, RP Sékaly
PLoS Pathog., 2017-12-21;13(12):e1006740.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
PD-1 status in CD8(+) T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer
Authors: BA Kansy, F Concha-Ben, RM Srivastava, HB Jie, G Shayan, Y Lei, J Moskovitz, J Moy, J Li, S Brandau, S Lang, NC Schmitt, GJ Freeman, WE Gooding, DA Clump, RL Ferris
Cancer Res., 2017-09-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study
Authors: J Hazeldine, DN Naumann, E Toman, D Davies, JRB Bishop, Z Su, P Hampson, RJ Dinsdale, N Crombie, NA Duggal, P Harrison, A Belli, JM Lord
PLoS Med., 2017-07-18;14(7):e1002338.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides
Authors: L Stenberg, M Stö beta el, G Ronchi, S Geuna, Y Yin, S Mommert, L Mårtensson, J Metzen, C Grothe, LB Dahlin, K Haastert-T
BMC Neurosci, 2017-07-18;18(1):53.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
pERK-dependent defective TCR-mediated activation of CD4(+) T cells in end-stage renal disease patients
Authors: L Huang, NHR Litjens, NM Kannegiete, M Klepper, CC Baan, MGH Betjes
Immun Ageing, 2017-06-19;14(0):14.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Natural killer cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study
Authors: D Rea, G Henry, Z Khaznadar, G Etienne, F Guilhot, F Nicolini, J Guilhot, P Rousselot, F Huguet, L Legros, M Gardembas, V Dubruille, A Guerci-Bre, A Charbonnie, F Maloisel, JC Ianotto, B Villemagne, FX Mahon, H Moins-Teis, N Dulphy, A Toubert
Haematologica, 2017-05-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
NKG2D ligand targeted Bispecific T Cell Engagers lead to robust antitumor activity against diverse human tumors
Authors: C Godbersen, TA Coupet, AM Huehls, T Zhang, MB Battles, JL Fisher, MS Ernstoff, CL Sentman
Mol. Cancer Ther., 2017-05-12;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Glatiramer Acetate, Dimethyl Fumarate, and Monomethyl Fumarate Upregulate the Expression of CCR10 on the Surface of Natural Killer Cells and Enhance Their Chemotaxis and Cytotoxicity
Authors: Zaidoon Al-Jaderi
Front Immunol, 2016-10-19;7(0):437.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tumor-infiltrating Tim-3(+) T cells proliferate avidly except when PD-1 is co-expressed: Evidence for intracellular cross talk
Authors: Robert L Ferris
Oncoimmunology, 2016-09-22;5(10):e1200778.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Glycolysis determines dichotomous regulation of T cell subsets in hypoxia
Authors: Yang Xu
J Clin Invest, 2016-06-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques
Authors: Hui Li
Proc Natl Acad Sci USA, 2016-05-31;113(24):E3413-22.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry -
Effective Cytotoxic T Lymphocyte Targeting of Persistent HIV-1 during Antiretroviral Therapy Requires Priming of Naive CD8+ T Cells
Authors: Kellie N Smith
MBio, 2016-05-31;7(3):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways.
Authors: Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S, Invernizzi P, Lugli E, Torzilli G, Gershwin M, Mavilio D
J Autoimmun, 2015-08-30;66(0):40-50.
Species: Human
Sample Types:
Applications: Flow Cytometry -
Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques.
Authors: Santra S, Tomaras G, Warrier R, Nicely N, Liao H, Pollara J, Liu P, Alam S, Zhang R, Cocklin S, Shen X, Duffy R, Xia S, Schutte R, Pemble Iv C, Dennison S, Li H, Chao A, Vidnovic K, Evans A, Klein K, Kumar A, Robinson J, Landucci G, Forthal D, Montefiori D, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb M, Michael N, Kim J, Soderberg K, Giorgi E, Blair L, Korber B, Moog C, Shattock R, Letvin N, Schmitz J, Moody M, Gao F, Ferrari G, Shaw G, Haynes B
PLoS Pathog, 2015-08-03;11(8):e1005042.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice.
Authors: Thomas S, Klobuch S, Podlech J, Plachter B, Hoffmann P, Renzaho A, Theobald M, Reddehase M, Herr W, Lemmermann N
PLoS Pathog, 2015-07-16;11(7):e1005049.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cytomegalovirus-Induced Expression of CD244 after Liver Transplantation Is Associated with CD8+ T Cell Hyporesponsiveness to Alloantigen.
Authors: de Mare-Bredemeijer E, Shi X, Mancham S, van Gent R, van der Heide-Mulder M, de Boer R, Heemskerk M, de Jonge J, van der Laan L, Metselaar H, Kwekkeboom J
J Immunol, 2015-07-13;195(4):1838-48.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function.
Authors: Wilson R, Ives M, Rao G, Lau A, Payne K, Kobayashi M, Arkwright P, Peake J, Wong M, Adelstein S, Smart J, French M, Fulcher D, Picard C, Bustamante J, Boisson-Dupuis S, Gray P, Stepensky P, Warnatz K, Freeman A, Rossjohn J, McCluskey J, Holland S, Casanova J, Uzel G, Ma C, Tangye S, Deenick E
J Exp Med, 2015-05-04;212(6):855-64.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Lymphocyte gene expression signatures from patients and mouse models of hereditary hemochromatosis reveal a function of HFE as a negative regulator of CD8+ T-lymphocyte activation and differentiation in vivo.
Authors: Costa M, Cruz E, Oliveira S, Benes V, Ivacevic T, Silva M, Vieira I, Dias F, Fonseca S, Goncalves M, Lima M, Leitao C, Muckenthaler M, Pinto J, Porto G
PLoS ONE, 2015-04-16;10(4):e0124246.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity.
Authors: Zaccard C, Watkins S, Kalinski P, Fecek R, Yates A, Salter R, Ayyavoo V, Rinaldo C, Mailliard R
J Immunol, 2014-12-29;194(3):1047-56.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Concurrent interaction of DCs with CD4(+) and CD8(+) T cells improves secondary CTL expansion: It takes three to tango.
Authors: Hoyer S, Prommersberger S, Pfeiffer I, Schuler-Thurner B, Schuler G, Dorrie J, Schaft N
Eur J Immunol, 2014-10-27;44(12):3543-59.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tetramer enrichment reveals the presence of phenotypically diverse hepatitis C virus-specific CD8+ T cells in chronic infection.
Authors: Nitschke K, Flecken T, Schmidt J, Gostick E, Marget M, Neumann-Haefelin C, Blum H, Price D, Thimme R
J Virol, 2014-10-15;89(1):25-34.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Exhaustion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders.
Authors: Perreau M, Vigano S, Bellanger F, Pellaton C, Buss G, Comte D, Roger T, Lacabaratz C, Bart P, Levy Y, Pantaleo G
J Exp Med, 2014-09-15;211(10):2033-45.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tracking in vivo dynamics of NK cells transferred in patients undergoing stem cell transplantation.
Authors: Killig M, Friedrichs B, Meisig J, Gentilini C, Bluthgen N, Loddenkemper C, Labopin M, Basara N, Pfrepper C, Niederwieser D, Uharek L, Romagnani C
Eur J Immunol, 2014-06-30;44(9):2822-34.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cytomegalovirus drives Vdelta2neg gammadelta T cell inflation in many healthy virus carriers with increasing age.
Authors: Alejenef A, Pachnio A, Halawi M, Christmas S, Moss P, Khan N
Clin Exp Immunol, 2014-06-01;176(3):418-28.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
TCR affinity associated with functional differences between dominant and subdominant SIV epitope-specific CD8+ T cells in Mamu-A*01+ rhesus monkeys.
Authors: Osuna C, Gonzalez A, Chang H, Hung A, Ehlinger E, Anasti K, Alam S, Letvin N
PLoS Pathog, 2014-04-17;10(4):e1004069.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
MART-1 peptide vaccination plus IMP321 (LAG-3Ig fusion protein) in patients receiving autologous PBMCs after lymphodepletion: results of a Phase I trial.
Authors: Romano E, Michielin O, Voelter V, Laurent J, Bichat H, Stravodimou A, Romero P, Speiser D, Triebel F, Leyvraz S, Harari A
J Transl Med, 2014-04-12;12(0):97.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses.
Authors: Clay C, Donart N, Fomukong N, Knight J, Overheim K, Tipper J, Van Westrienen J, Hahn F, Harrod K
Immun Ageing, 2014-03-19;11(1):4.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Tissue Homogenates
Applications: Flow Cytometry -
IL-27 in human secondary lymphoid organs attracts myeloid dendritic cells and impairs HLA class I-restricted antigen presentation.
Authors: Morandi, Fabio, Di Carlo, Emma, Ferrone, Soldano, Petretto, Andrea, Pistoia, Vito, Airoldi, Irma
J Immunol, 2014-02-19;192(6):2634-42.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential.
Authors: Lehmann, Dorit, Spanholtz, Jan, Sturtzel, Caterina, Tordoir, Marleen, Schlechta, Bernhard, Groenewegen, Dirk, Hofer, Erhard
PLoS ONE, 2014-01-31;9(1):e87131.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Peripheral blood CD56(bright) NK cells respond to stem cell factor and adhere to its membrane-bound form after upregulation of c-kit.
Authors: Pradier A, Tabone-Eglinger S, Huber V, Bosshard C, Rigal E, Wehrle-Haller B, Roosnek E
Eur J Immunol, 2013-11-20;44(2):511-20.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Both HLA-B*57 and plasma HIV RNA levels contribute to the HIV-specific CD8+ T cell response in HIV controllers.
Authors: Lecuroux C, Saez-Cirion A, Girault I, Versmisse P, Boufassa F, Avettand-Fenoel V, Rouzioux C, Meyer L, Pancino G, Lambotte O, Sinet M, Venet A
J Virol, 2013-10-16;88(1):176-87.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
IL-18-based combinatorial adjuvants promote the intranodal production of CCL19 by NK cells and dendritic cells of cancer patients.
Authors: Wong J, Muthuswamy R, Bartlett D, Kalinski P
Oncoimmunology, 2013-09-12;2(9):e26245.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Bone marrow T cells from the femur are similar to iliac crest derived cells in old age and represent a useful tool for studying the aged immune system.
Authors: Pritz T, Landgraf-Rauf K, Herndler-Brandstetter D, Rauf R, Lair J, Gassner R, Weinberger B, Krismer M, Grubeck-Loebenstein B
Immun Ageing, 2013-05-04;10(1):17.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CCR5 is a receptor for Staphylococcus aureus leukotoxin ED.
Authors: Alonzo, Francis, Kozhaya, Lina, Rawlings, Stephen, Reyes-Robles, Tamara, DuMont, Ashley L, Myszka, David G, Landau, Nathanie, Unutmaz, Derya, Torres, Victor J
Nature, 2012-12-12;493(7430):51-5.
Species: Human
Sample Types: Whole Cells
-
The CD46-Jagged1 interaction is critical for human TH1 immunity.
Authors: Le Friec G, Sheppard D, Whiteman P, Karsten C, Shamoun S, Laing A, Bugeon L, Dallman M, Melchionna T, Chillakuri C, Smith R, Drouet C, Couzi L, Fremeaux-Bacchi V, Kohl J, Waddington S, McDonnell J, Baker A, Handford P, Lea S, Kemper C
Nat Immunol, 2012-10-21;13(12):1213-21.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CCR2+CCR5+ T Cells Produce Matrix Metalloproteinase-9 and Osteopontin in the Pathogenesis of Multiple Sclerosis.
Authors: Sato W, Tomita A, Ichikawa D, Lin Y, Kishida H, Miyake S, Ogawa M, Okamoto T, Murata M, Kuroiwa Y, Aranami T, Yamamura T
J Immunol, 2012-10-15;189(10):5057-65.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Biomarkers on melanoma patient T cells associated with ipilimumab treatment.
Authors: Wang W, Yu D, Sarnaik A, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber J
J Transl Med, 2012-07-12;10(0):146.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation.
Authors: Napoletano C, Zizzari IG, Rughetti A, Rahimi H, Irimura T, Clausen H, Wandall HH, Belleudi F, Bellati F, Pierelli L, Frati L, Nuti M
Eur. J. Immunol., 2012-04-01;42(4):936-45.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Identification of human NK17/NK1 cells.
Authors: Pandya AD, Al-Jaderi Z, Hoglund RA, Holmoy T, Harbo HF, Norgauer J, Maghazachi AA
PLoS ONE, 2011-10-21;6(10):e26780.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Primary B-cell deficiencies reveal a link between human IL-17-producing CD4 T-cell homeostasis and B-cell differentiation.
Authors: Barbosa RR, Silva SP, Silva SL, Melo AC, Pedro E, Barbosa MP, Pereira-Santos MC, Victorino RM, Sousa AE
PLoS ONE, 2011-08-03;6(8):e22848.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo.
Authors: Bosco MC, Pierobon D, Blengio F, Raggi F, Vanni C, Gattorno M, Eva A, Novelli F, Cappello P, Giovarelli M, Varesio L
Blood, 2010-12-10;117(9):2625-39.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response.
Authors: Zhang HH, Song K, Rabin RL, Hill BJ, Perfetto SP, Roederer M, Douek DC, Siegel RM, Farber JM
J. Immunol., 2010-10-27;185(11):6646-63.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells.
Authors: Jones GW, McLoughlin RM, Hammond VJ, Parker CR, Williams JD, Malhotra R, Scheller J, Williams AS, Rose-John S, Topley N, Jones SA
J. Immunol., 2010-01-18;184(4):2130-9.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Expression of soluble vascular endothelial growth factor receptor-1 in human monocyte-derived mature dendritic cells contributes to their antiangiogenic property.
Authors: Kishuku M, Nishioka Y, Abe S, Kishi J, Ogino H, Aono Y, Azuma M, Kinoshita K, Batmunkh R, Rentsenhand B, Makino H, Ranjan P, Minakuchi K, Sone S
J. Immunol., 2009-12-15;183(12):8176-85.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CCL21 overexpressed on lymphatic vessels drives thymic hyperplasia in myasthenia.
Authors: Berrih-Aknin S, Ruhlmann N, Bismuth J, Cizeron-Clairac G, Zelman E, Shachar I, Dartevelle P, de Rosbo NK, Le Panse R
Ann. Neurol., 2009-10-01;66(4):521-31.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Changes in chemokines and chemokine receptor expression on tonsillar B cells upon Epstein-Barr virus infection.
Authors: Ehlin-Henriksson B, Liang W, Cagigi A, Mowafi F, Klein G, Nilsson A
Immunology, 2009-08-01;127(4):549-57.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Phenotypic and functional markers for 1alpha,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells.
Authors: Pedersen AW, Holmstrom K, Jensen SS, Fuchs D, Rasmussen S, Kvistborg P, Claesson MH, Zocca MB
Clin. Exp. Immunol., 2009-07-01;157(1):48-59.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation.
Authors: Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, Carmagnat M, Loiseau P, Tamouza R, Scieux C, Rabian C, Di Santo JP, Charron D, Janin A, Socie G, Toubert A
J. Immunol., 2008-08-01;181(3):2227-37.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Intravenous immunoglobulins suppress T-cell priming by modulating the bidirectional interaction between dendritic cells and natural killer cells.
Authors: Tha-In T, Metselaar HJ, Tilanus HW, Groothuismink ZM, Kuipers EJ, De Man RA, Kwekkeboom J
Blood, 2007-08-02;110(9):3253-62.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis.
Authors: Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, Nestle FO
Nat. Med., 2007-07-01;13(7):836-42.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection.
Authors: Fluur C, De Milito A, Fry TJ, Vivar N, Eidsmo L, Atlas A, Federici C, Matarrese P, Logozzi M, Rajnavolgyi E, Mackall CL, Fais S, Chiodi F, Rethi B
J. Immunol., 2007-04-15;178(8):5340-50.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Aberration of CCR7 CD8 memory T cells from patients with systemic lupus erythematosus: an inducer of T helper type 2 bias of CD4 T cells.
Authors: Sen Y, Chunsong H, Baojun H, Linjie Z, Qun L, San J, Qiuping Z, Junyan L, Zhang X, Jinquan T
Immunology, 2004-06-01;112(2):274-89.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CCR7 Fluorescein-conjugated Antibody
There are currently no reviews for this product. Be the first to review Human CCR7 Fluorescein-conjugated Antibody and earn rewards!
Have you used Human CCR7 Fluorescein-conjugated Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image




