Human CXCR4 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
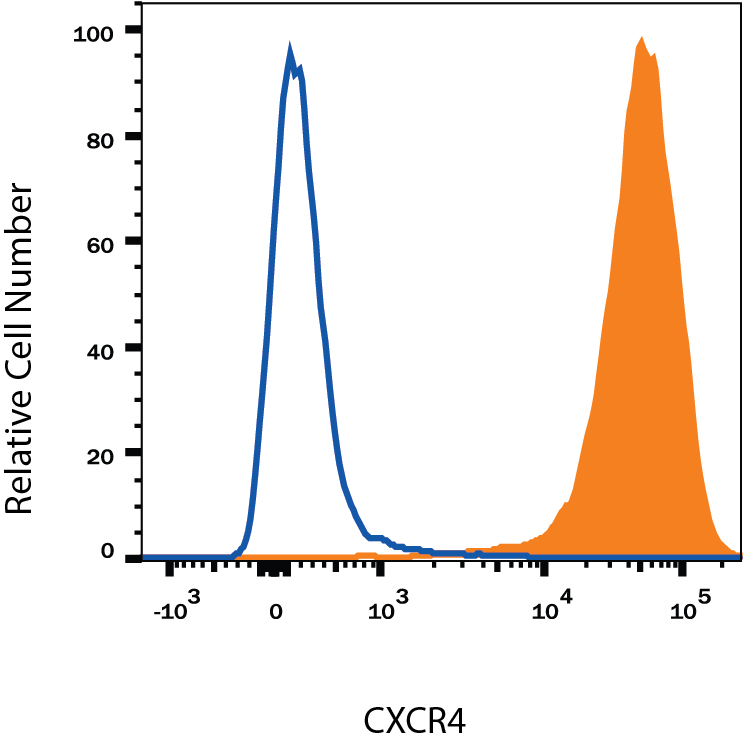
Detection of CXCR4 in Jurkat Human Cell Line by Flow Cytometry. Jurkat human acute T cell leukemia cell line was stained with Mouse Anti-Human CXCR4 Monoclonal Antibody (Catalog # MAB172, filled histogram) or isotype control antibody (Catalog # MAB004, open histogram), followed by Allophycocyanin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0101B). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
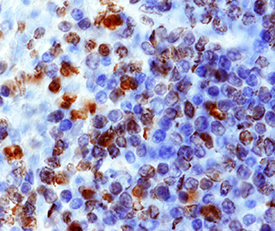
CXCR4 in Human Lymph Node. CXCR4 was detected in immersion fixed paraffin-embedded sections of human lymph node using 15 µg/mL Human CXCR4 Monoclonal Antibody (Catalog # MAB172) overnight at 4 °C. Tissue was stained with the Anti-Mouse HRP-AEC Cell & Tissue Staining Kit (red; Catalog # CTS003) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
CXCR4 in Human Spleen. CXCR4 was detected in immersion fixed paraffin-embedded sections of human spleen using Mouse Anti-Human CXCR4 Monoclonal Antibody (Catalog # MAB172) at 15 µg/mL overnight at 4 °C. Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm and nuclei. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Chemotaxis Induced by CXCL12/SDF‑1 alpha and Neutralization by Human CXCR4 Antibody. Recombinant Human/Feline/Rhesus Macaque CXCL12/SDF-1a (Catalog # 350-NS) chemoattracts the BaF3 mouse pro-B cell line transfected with human CXCR4 in a dose-dependent manner (orange line). The amount of cells that migrated through to the lower chemotaxis chamber was measured by Resazurin (Catalog # AR002). Chemotaxis elicited by Recombinant Human/Feline/Rhesus Macaque CXCL12/SDF-1a (1 ng/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-Human CXCR4 Monoclonal Antibody (Catalog # MAB172). The ND50 is typically 2.5-12 µg/mL.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CXCR4
CXCR4, also known as CD184, is a G-protein-linked seven transmembrane spanning receptor that binds stromal cell-derived factor-1 (SDF-1). CXCR4 acts as a co-factor for T-cell tropic HIV-1 and -2 viral entry into cells. While primarily a membrane protein, CXCR4 undergoes trafficking and internalization in response to stimulation with phorbol esters and ligand (1). Cytoplasmic and nuclear localization of CXCR4 has been observed in colorectal and renal carcinomas (2,3) and it has been used as the basis of prognosis and metastatic state (3,4,5).
- Orsini, M.J. et al. (1999) J. Biol. Chem. 274:31076.
- Zagzag, D. et al. (2005) Cancer Res. 65:6178.
- Speetjens, F.M. et al. (2009) Cancer Microenvironment 2:1.
- Wang, L. et al. (2009) Oncology Reports 22:1333.
- Amara, S. et al. (2015) Cancer Biomark. 15:869.
Product Datasheets
Citations for Human CXCR4 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
55
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
CXCR4-CXCL12-CXCR7 and PD-1/PD-L1 in Pancreatic Cancer: CXCL12 Predicts Survival of Radically Resected Patients
Authors: C D'Alterio, A Giardino, G Scognamigl, G Butturini, L Portella, G Guardascio, I Frigerio, M Montella, S Gobbo, G Martignoni, V Napolitano, F De Vita, F Tatangelo, R Franco, S Scala
Cells, 2022-10-22;11(21):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Analysis of the chemotactic factors for tumor-infiltrating fibrocytes and their prognostic significances in lung cancer
Authors: M Tobiume, A Mitsuhashi, A Saijo, H Ogino, T Afroj, H Ogawa, H Goto, S Sato, A Abe, K Haji, R Ozaki, H Takizawa, Y Nishioka
Oncology Letters, 2022-09-30;24(5):417.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients
Authors: C D'Alterio, A Spina, L Arenare, P Chiodini, M Napolitano, F Galdiero, L Portella, V Simeon, S Signoriell, F Raspaglies, D Lorusso, C Pisano, N Colombo, GF Zannoni, NS Losito, R De Cecio, G Scognamigl, D Califano, D Russo, V Tuninetti, MC Piccirillo, P Gargiulo, F Perrone, S Pignata, S Scala
Cancers, 2022-04-06;14(7):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Combination of IDO1high and CCL19low expression in the tumor tissue reduces survival in HPV positive cervical cancer
Authors: H Jayakumar, A Seetharama, S Sunder Sin, H Dhandapani, J Subramani, S Ganeshraja, R Thangaraja, P Ramanathan
Journal of reproductive immunology, 2021-11-23;149(0):103454.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Role of CCR3 in respiratory syncytial virus infection of airway epithelial cells.
Authors: Wellemans V, Benhassou H, Fuselier E, Bellesort F, Dury S, Lebargy F, Dormoy V, Fichel C, Naour R, Gounni A, Lamkhioued B
iScience, 2021-11-14;24(12):103433.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Mechanisms of HIV-1 evasion to the antiviral activity of chemokine CXCL12 indicate potential links with pathogenesis
Authors: M Armani-Tou, Z Zhou, R Gasser, I Staropoli, V Cantaloube, Y Benureau, J Garcia-Per, M Pérez-Olme, V Lorin, B Puissant-L, L Assoumou, C Delaugerre, JD Lelièvre, Y Lévy, H Mouquet, G Martin-Blo, J Alcami, F Arenzana-S, J Izopet, P Colin, B Lagane
PloS Pathogens, 2021-04-19;17(4):e1009526.
Species: Human
Sample Types: Whole Cells
Applications: Binding Assay -
Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer's disease
Authors: A McQuade, YJ Kang, J Hasselmann, A Jairaman, A Sotelo, M Coburn, SK Shabestari, JP Chadarevia, G Fote, CH Tu, E Danhash, J Silva, E Martinez, C Cotman, GA Prieto, LM Thompson, JS Steffan, I Smith, H Davtyan, M Cahalan, H Cho, M Blurton-Jo
Nat Commun, 2020-10-23;11(1):5370.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Soluble Compounds Released by Hypoxic Stroma Confer Invasive Properties to Pancreatic Ductal Adenocarcinoma
Authors: D Liu, A Steins, R Klaassen, AP van der Za, RJ Bennink, G van Tienho, MG Besselink, MF Bijlsma, HWM van Laarho
Biomedicines, 2020-10-22;8(11):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Involvement of CXCL12/CXCR4 in the motility of human first-trimester endometrial epithelial cells through an autocrine mechanism by activating PI3K/AKT signaling
Authors: J Zheng, D Qu, C Wang, L Ding, W Zhou
BMC Pregnancy Childbirth, 2020-02-10;20(1):87.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC, Neutralization -
High CXCR4 Expression Predicts a Poor Prognosis in Resected Lung Adenosquamous Carcinoma
Authors: Q Zhu, R Luo, J Gu, Y Hou, Z Chen, F Xu, L Wang, W Mao, C Lu, D Ge
J Cancer, 2020-01-01;11(4):810-818.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Clinicopathologic determinants of pathologic treatment response in neoadjuvant treated rectal adenocarcinoma
Authors: I González, PS Bauer, WC Chapman, Z Alipour, R Rais, J Liu, D Chatterjee
Ann Diagn Pathol, 2019-12-14;45(0):151452.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Chemokine Receptor Expression Pattern Correlates to Progression of Conjunctival Melanocytic Lesions
Authors: JA van Ipenbu, NE de Waard, NC Naus, MJ Jager, D Paridaens, RM Verdijk
Invest. Ophthalmol. Vis. Sci., 2019-07-01;60(8):2950-2957.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Clinical impact of different exosomes' protein expression in pancreatic ductal carcinoma patients treated with standard first line palliative chemotherapy
Authors: R Giampieri, F Piva, G Occhipinti, A Bittoni, A Righetti, S Pagliarett, A Murrone, F Bianchi, C Amantini, M Giulietti, G Ricci, G Principato, G Santoni, R Berardi, S Cascinu
PLoS ONE, 2019-05-02;14(5):e0215990.
Species: Human
Sample Types: Plasma
Applications: ELISA Detection -
Breast Cancer: An Examination of the Potential of ACKR3 to Modify the Response of CXCR4 to CXCL12
Authors: I Del Molino, GC Wilkins, A Meeson, S Ali, JA Kirby
Int J Mol Sci, 2018-11-14;19(11):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Senescent fibroblasts drive ageing pigmentation: ?A potential therapeutic target for senile lentigo
Authors: JE Yoon, Y Kim, S Kwon, M Kim, YH Kim, JH Kim, TJ Park, HY Kang
Theranostics, 2018-09-09;8(17):4620-4632.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice
Authors: MJ Chau, TC Deveau, X Gu, YS Kim, Y Xu, SP Yu, L Wei
BMC Neurosci, 2018-04-12;19(1):20.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Tumor Microenvironment in Functional Adrenocortical Adenomas: Immune Cell Infiltration in Cortisol-producing Adrenocortical Adenoma
Authors: Y Kitawaki, Y Nakamura, F Kubota-Nak, Y Yamazaki, Y Miki, S Hata, K Ise, K Kikuchi, R Morimoto, F Satoh, H Sasano
Hum. Pathol., 2018-03-27;0(0):.
Species: Human
Sample Types: WholeTissue
Applications: IHC-P -
Expression of CXC-motif-chemokine 12 and the receptor C-X-C receptor 4 in glioma and theeffect on peritumoral brain edema
Authors: W Tang, Y Chen, X Wang, Y Chen, J Zhang, Z Lin
Oncol Lett, 2017-12-08;15(2):2501-2507.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Mechanisms by which CXCR4/CXCL12 cause metastatic behavior in pancreatic cancer
Authors: J Zhang, C Liu, X Mo, H Shi, S Li
Oncol Lett, 2017-12-05;15(2):1771-1776.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Cell-based therapy using miR-302-367 expressing cells represses glioblastoma growth
Authors: M Fareh, F Almairac, L Turchi, F Burel-Vand, P Paquis, D Fontaine, S Lacas-Gerv, MP Junier, H Chneiweiss, T Virolle
Cell Death Dis, 2017-03-30;8(3):e2713.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Cathepsin K cleavage of SDF-1? inhibits its chemotactic activity towards glioblastoma stem-like cells
Authors: Vashendriya V V Hira
Biochim. Biophys. Acta, 2016-12-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The Glycosylphosphatidylinositol-Anchored Variable Region of Llama Heavy Chain-Only Antibody JM4 Efficiently Blocks both Cell-Free and T Cell-T Cell Transmission of Human Immunodeficiency Virus Type 1
Authors: L Liu, W Wang, J Matz, C Ye, L Bracq, J Delon, JT Kimata, Z Chen, S Benichou, P Zhou
J. Virol., 2016-11-14;90(23):10642-10659.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Signal transmission through the CXC chemokine receptor 4 (CXCR4) transmembrane helices
Proc Natl Acad Sci USA, 2016-08-19;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: IHC-Fr -
Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model.
Authors: Mercurio L, Ajmone-Cat M, Cecchetti S, Ricci A, Bozzuto G, Molinari A, Manni I, Pollo B, Scala S, Carpinelli G, Minghetti L
J Exp Clin Cancer Res, 2016-03-25;35(0):55.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Simultaneous Activation of Induced Heterodimerization between CXCR4 Chemokine Receptor and Cannabinoid Receptor 2 (CB2) Reveals a Mechanism for Regulation of Tumor Progression
Authors: CJ Coke, KA Scarlett, MA Chetram, KJ Jones, BJ Sandifer, AS Davis, AI Marcus, CV Hinton
J. Biol. Chem., 2016-02-03;291(19):9991-10005.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Identification and Characterization of CXCR4-Positive Gastric Cancer Stem Cells.
Authors: Fujita T, Chiwaki F, Takahashi R, Aoyagi K, Yanagihara K, Nishimura T, Tamaoki M, Komatsu M, Komatsuzaki R, Matsusaki K, Ichikawa H, Sakamoto H, Yamada Y, Fukagawa T, Katai H, Konno H, Ochiya T, Yoshida T, Sasaki H
PLoS ONE, 2015-06-25;10(6):e0130808.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
The long pentraxin PTX3 promotes fibrocyte differentiation.
Authors: Pilling D, Cox N, Vakil V, Verbeek J, Gomer R
PLoS ONE, 2015-03-16;10(3):e0119709.
Species: Human
Sample Types: Whole Cells
Applications: IHC-Fr -
CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and mediate the invasion of lung cancer cell lines in vitro.
Authors: Hu, Weidong, Liu, Yue, Zhou, Wenhui, Si, Lianlian, Ren, Liang
PLoS ONE, 2014-06-04;9(6):e99056.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer.
Authors: Hartmann N, Giese N, Giese T, Poschke I, Offringa R, Werner J, Ryschich E
Clin Cancer Res, 2014-04-24;20(13):3422-33.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms.
Authors: Jarajapu Y, Hazra S, Segal M, Li Calzi S, Jadhao C, Qian K, Mitter S, Raizada M, Boulton M, Grant M
PLoS ONE, 2014-04-08;9(4):e93965.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
An important role of the SDF-1/CXCR4 axis in chronic skin inflammation.
Authors: Zgraggen, Silvana, Huggenberger, Reto, Kerl, Katrin, Detmar, Michael
PLoS ONE, 2014-04-02;9(4):e93665.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Lymphatic specific disruption in the fine structure of heparan sulfate inhibits dendritic cell traffic and functional T cell responses in the lymph node.
Authors: Yin X, Johns S, Kim D, Mikulski Z, Salanga C, Handel T, Macal M, Zuniga E, Fuster M
J Immunol, 2014-02-03;192(5):2133-42.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
PCaAnalyser: a 2D-image analysis based module for effective determination of prostate cancer progression in 3D culture.
Authors: Hoque, Md Tamji, Windus, Louisa C, Lovitt, Carrie J, Avery, Vicky M
PLoS ONE, 2013-11-20;8(11):e79865.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Expression of the chemokine receptor CXCR7 in CXCR4-expressing human 143B osteosarcoma cells enhances lung metastasis of intratibial xenografts in SCID mice.
Authors: Brennecke P, Arlt M, Muff R, Campanile C, Gvozdenovic A, Husmann K, Holzwarth N, Cameroni E, Ehrensperger F, Thelen M, Born W, Fuchs B
PLoS ONE, 2013-09-10;8(9):e74045.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis.
Authors: Gassenmaier M, Chen D, Buchner A, Henkel L, Schiemann M, Mack B, Schendel D, Zimmermann W, Pohla H
Stem Cells, 2013-08-01;31(8):1467-76.
Species: Human
Sample Types: Whole Cells
Applications: IHC-P -
Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070.
Authors: O'Boyle, G, Swidenbank, I, Marshall, H, Barker, C E, Armstrong, J, White, S A, Fricker, S P, Plummer, R, Wright, M, Lovat, P E
Br J Cancer, 2013-03-28;108(8):1634-40.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma.
Authors: Zhang, Lin, Ye, Shu-Biao, Ma, Gang, Tang, Xiao-Fen, Chen, Shi-Ping, He, Jia, Liu, Wan-Li, Xie, Dan, Zeng, Yi-Xin, Li, Jiang
J Transl Med, 2013-03-08;11(0):60.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer.
Authors: Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, Tacheuchi S, Mouri H, Yamashita K, Ohtsubo K, Yano S
Clin. Cancer Res., 2011-04-11;17(11):3619-30.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Intratumoral induction of CD103 triggers tumor-specific CTL function and CCR5-dependent T-cell retention.
Authors: Franciszkiewicz K, Le Floc'h A, Jalil A, Vigant F, Robert T, Vergnon I, Mackiewicz A, Benihoud K, Validire P, Chouaib S, Combadiere C, Mami-Chouaib F
Cancer Res., 2009-07-28;69(15):6249-55.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Alternative implication of CXCR4 in JAK2/STAT3 activation in small cell lung cancer.
Authors: Pfeiffer M, Hartmann TN, Leick M, Catusse J, Schmitt-Graeff A, Burger M
Br. J. Cancer, 2009-05-19;100(12):1949-56.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells.
Authors: Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P
Dev. Biol., 2009-04-07;330(2):286-304.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Clinical and biological significance of CXCL12 and CXCR4 expression in adult testes and germ cell tumours of adults and adolescents.
Authors: Gilbert DC, Chandler I, McIntyre A, Goddard NC, Gabe R, Huddart RA, Shipley J
J. Pathol., 2009-01-01;217(1):94-102.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
Regulation of CXCR4 by the Notch ligand delta-like 4 in endothelial cells.
Authors: Williams CK, Segarra M, Sierra Mde L, Sainson RC, Tosato G, Harris AL
Cancer Res., 2008-03-15;68(6):1889-95.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts.
Authors: Tait LR, Pauley RJ, Santner SJ, Heppner GH, Heng HH, Rak JW, Miller FR
Int. J. Cancer, 2007-05-15;120(10):2127-34.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis.
Authors: Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P
J. Immunol., 2006-05-15;176(10):5735-9.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF.
Authors: Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, Minatoguchi S, Fujiwara T, Fujiwara H
Cardiovasc. Res., 2006-05-09;71(3):455-65.
Species: Rabbit
Sample Types: Whole Tissue
Applications: IHC-P -
Human melanoma metastases express functional CXCR4.
Authors: Scala S, Giuliano P, Ascierto PA, Ierano C, Franco R, Napolitano M, Ottaiano A, Lombardi ML, Luongo M, Simeone E, Castiglia D, Mauro F, De Michele I, Calemma R, Botti G, Caraco C, Nicoletti G, Satriano RA, Castello G
Clin. Cancer Res., 2006-04-15;12(8):2427-33.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr, IHC-P -
Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis.
Authors: Haringman JJ, Smeets TJ, Reinders-Blankert P
Ann. Rheum. Dis., 2005-08-17;65(3):294-300.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus.
Authors: Meller S, Winterberg F, Gilliet M, Muller A, Lauceviciute I, Rieker J, Neumann NJ, Kubitza R, Gombert M, Bunemann E, Wiesner U, Franken-Kunkel P, Kanzler H, Dieu-Nosjean MC, Amara A, Ruzicka T, Lehmann P, Zlotnik A, Homey B
Arthritis Rheum., 2005-05-01;52(5):1504-16.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone.
Authors: Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ, Lee FY
J. Orthop. Res., 2005-01-01;23(1):203-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer.
Authors: Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, Masui T, Fujimoto K, Tamamura H, Hiramatsu K, Fujii N, Imamura M
Mol. Cancer Ther., 2004-01-01;3(1):29-37.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells.
Authors: Zhang Y, Foudi A, Geay JF, Berthebaud M, Buet D, Jarrier P, Jalil A, Vainchenker W, Louache F
Stem Cells, 2004-01-01;22(6):1015-29.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin.
Authors: Cardones AR, Murakami T, Hwang ST
Cancer Res., 2003-10-15;63(20):6751-7.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CXCR4 function requires membrane cholesterol: implications for HIV infection.
Authors: Nguyen DH, Taub D
J. Immunol., 2002-04-15;168(8):4121-6.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire.
Authors: Campbell J, Qin S, Unutmaz D, Soler D, Murphy K, Hodge M, Wu L, Butcher E
J Immunol, 2001-06-01;166(11):6477-82.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CXCR4 Antibody
Average Rating: 5 (Based on 4 Reviews)
Have you used Human CXCR4 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Antibody was printed on custom arrays and incubated with fluorescently labeled human EDTA plasma
Cell line stained with human CXCR4 for 45 min and secondary antibody anti-mouse FITC for 45min & Isotype control and unstained cell used in experiment









