Human/Mouse E-Cadherin Antibody Summary
Asp157-Val709
Accession # P09803
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
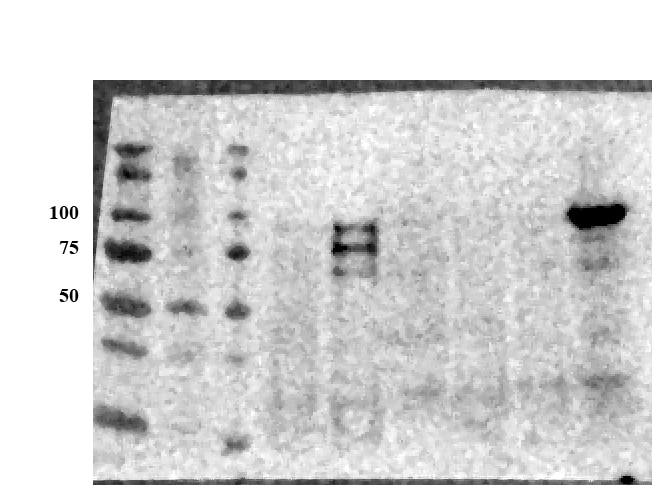
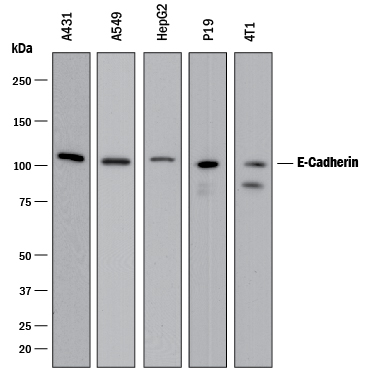
Detection of Human and Mouse E‑Cadherin by Western Blot. Western blot shows lysates of A431 human epithelial carcinoma cell line, A549 human lung carcinoma cell line, HepG2 human hepatocellular carcinoma cell line, P19 mouse embryonal carcinoma cell line, and 4T1 mouse breast cancer cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for E-Cadherin at approximately 110 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
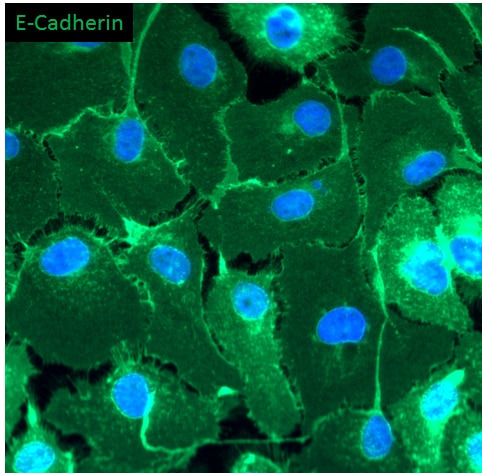
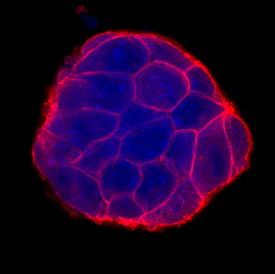
E‑Cadherin in D3 Mouse Embryonic Stem Cell Line. E-Cadherin was detected in immersion fixed D3 mouse embryonic stem cell line using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Stem Cells on Coverslips.
 View Larger
View Larger
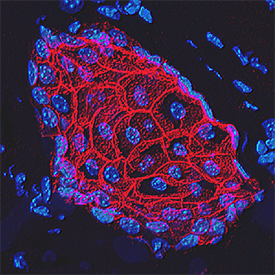
E‑Cadherin in Mouse Skin. E-Cadherin was detected in perfusion fixed frozen sections of mouse skin using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 1.7 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to plasma membranes in keratinocytes. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
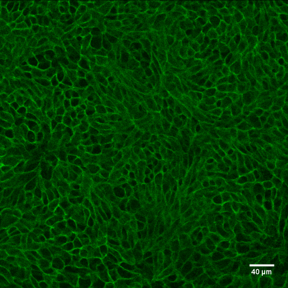
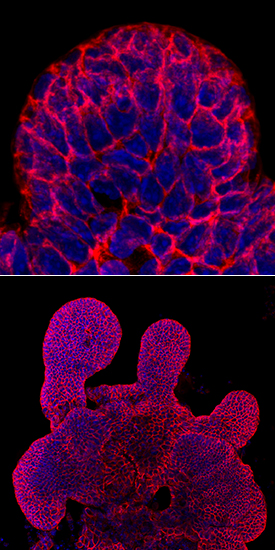
E‑Cadherin in Mouse Intestinal Organoids. E-Cadherin was detected in immersion fixed mouse intestinal organoids using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (green; Catalog # NL001) and counterstained with DAPI (blue). Magnification shown at 100X (upper panel) and 40X (lower panel). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
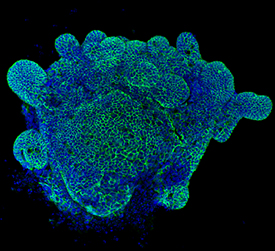 View Larger
View Larger
E‑Cadherin in Mouse Intestinal Organoids. E-Cadherin was detected in immersion fixed mouse intestinal organoids using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Goat IgG Secondary Antibody (green; Catalog # NL003) and counterstained with DAPI (blue). Specific staining was localized to cell surfaces. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
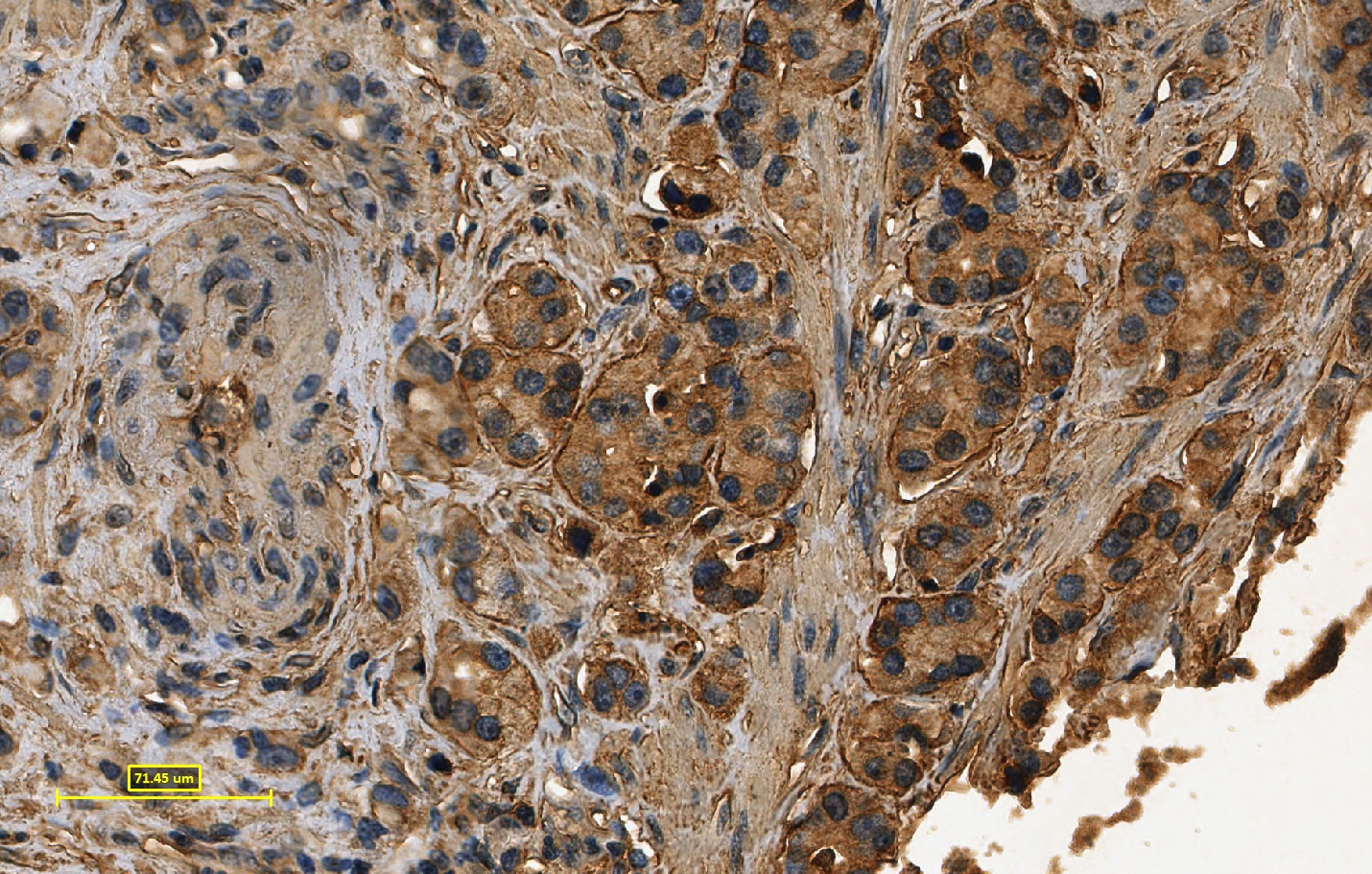
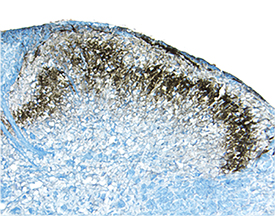
E‑Cadherin in Mouse Spinal Cord. E-Cadherin was detected in perfusion fixed frozen sections of mouse spinal cord using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) at 1.7 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to dorsal horn. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
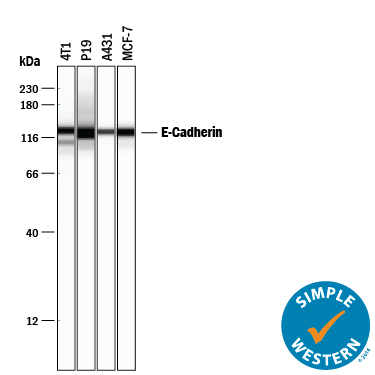
Detection of Human and Mouse E‑Cadherin by Simple WesternTM. Simple Western lane view shows lysates of 4T1 mouse breast cancer cell line, P19 mouse embryonal carcinoma cell line, A431 human epithelial carcinoma cell line, and MCF-7 human breast cancer cell line, loaded at 0.2 mg/mL. A specific band was detected for E-Cadherin at approximately 128 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF748) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: E-Cadherin
Epithelial (E)‑Cadherin (ECAD), also known as cell-CAM120/80 in the human, uvomorulin in the mouse, Arc-1 in the dog, and L-CAM in the chicken, is a member of the cadherin family of cell adhesion molecules. Cadherins are calcium-dependent transmembrane proteins, which bind to one another in a homophilic manner. On their cytoplasmic side, they associate with the three catenins, alpha, beta, and gamma (plakoglobin). This association links the cadherin protein to the cytoskeleton. Without association with the catenins, the cadherins are non-adhesive. Cadherins play a role in development, specifically in tissue formation. They may also help to maintain tissue architecture in the adult. E-Cadherin may also play a role in tumor development, as loss of E-Cadherin has been associated with tumor invasiveness. E-Cadherin is a classical cadherin molecule. Classical cadherins consist of a large extracellular domain which contains DXD and DXNDN repeats responsible for mediating calcium‑dependent adhesion, a single-pass transmembrane domain, and a short carboxy-terminal cytoplasmic domain responsible for interacting with the catenins. E‑Cadherin contains five extracellular calcium-binding domains of approximately 110 amino acids each.
- Bussemakers, M.J.G. et al. (1993) Mol. Biol. Reports 17:123.
- Overduin, M. et al. (1995) Science 267:386.
- Takeichi, M. (1991) 251:1451.
Product Datasheets
Citations for Human/Mouse E-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
78
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Distinct expression patterns of Hedgehog signaling components in mouse gustatory system during postnatal tongue development and adult homeostasis
Authors: Kumari, A;Franks, NE;Li, L;Audu, G;Liskowicz, S;Johnson, JD;Mistretta, CM;Allen, BL;
PloS one
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Trans-omic profiling uncovers molecular controls of early human cerebral organoid formation
Authors: Chen, C;Lee, S;Zyner, KG;Fernando, M;Nemeruck, V;Wong, E;Marshall, LL;Wark, JR;Aryamanesh, N;Tam, PPL;Graham, ME;Gonzalez-Cordero, A;Yang, P;
Cell reports
Species: Human
Sample Types: Organoid
Applications: Immunohistochemistry -
Venous-plexus-associated lymphoid hubs support meningeal humoral immunity
Authors: Fitzpatrick, Z;Ghabdan Zanluqui, N;Rosenblum, JS;Tuong, ZK;Lee, CYC;Chandrashekhar, V;Negro-Demontel, ML;Stewart, AP;Posner, DA;Buckley, M;Allinson, KSJ;Mastorakos, P;Chittiboina, P;Maric, D;Donahue, D;Helmy, A;Tajsic, T;Ferdinand, JR;Portet, A;Peñalver, A;Gillman, E;Zhuang, Z;Clatworthy, MR;McGavern, DB;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Delivery of a BET protein degrader via a CEACAM6-targeted antibody-drug conjugate inhibits tumour growth in pancreatic cancer models
Authors: Nakazawa, Y;Miyano, M;Tsukamoto, S;Kogai, H;Yamamoto, A;Iso, K;Inoue, S;Yamane, Y;Yabe, Y;Umihara, H;Taguchi, J;Akagi, T;Yamaguchi, A;Koga, M;Toshimitsu, K;Hirayama, T;Mukai, Y;Machinaga, A;
Nature communications
Species: Murine polyomavirus strain A3
Sample Types: Organoid, Whole Tissue
Applications: Immunohistochemistry -
Measurement of adhesion and traction of cells at high yield (MATCHY) reveals an energetic ratchet driving nephron condensation
Authors: Liu, J;Prahl, LS;Huang, A;Hughes, AJ;
bioRxiv : the preprint server for biology
Species: Murine polyomavirus strain A3
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Intestinal transit-amplifying cells require METTL3 for growth factor signaling and cell survival
Authors: Danan, CH;Naughton, KE;Hayer, KE;Vellappan, S;McMillan, EA;Zhou, Y;Matsuda, R;Nettleford, SK;Katada, K;Parham, LR;Ma, X;Chowdhury, A;Wilkins, BJ;Shah, P;Weitzman, MD;Hamilton, KE;
JCI insight
Species: Transgenic Mouse
Sample Types: Organoid
Applications: Immunohistochemistry -
VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation
Authors: Mapunda JA, Pareja J, Vladymyrov M et al.
Nat Commun
-
Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
Authors: Tixi W, Maldonado M, Chang YT et al.
eLife
-
An inducible hACE2 transgenic mouse model recapitulates SARS-CoV-2 infection and pathogenesis in vivo
Authors: Liu, K;Tang, M;Xu, W;Meng, X;Jin, H;Han, M;Pu, J;Li, Y;Jiao, F;Sun, R;Shen, R;Lui, KO;Lu, L;Zhou, B;
Proceedings of the National Academy of Sciences of the United States of America
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Opposing roles for TGF?- and BMP-signaling during nascent alveolar differentiation in the developing human lung
Authors: Frum, T;Hsu, PP;Hein, RFC;Conchola, AS;Zhang, CJ;Utter, OR;Anand, A;Zhang, Y;Clark, SG;Glass, I;Sexton, JZ;Spence, JR;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
IGFBP2 expressing midlobular hepatocytes preferentially contribute to liver homeostasis and regeneration
Authors: Lin, YH;Wei, Y;Zeng, Q;Wang, Y;Pagani, CA;Li, L;Zhu, M;Wang, Z;Hsieh, MH;Corbitt, N;Zhang, Y;Sharma, T;Wang, T;Zhu, H;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Non-optimal bacteria species induce neutrophil-driven inflammation and barrier disruption in the female genital tract
Authors: M Costa Fuji, A Yazdanpana, S Horne, A Lamont, P Lopez, C Farr Zuend, K Birse, M Taverner, R Greenslade, M Abou, L Noel-Romas, B Abrenica, O Ajibola, N Ikeogu, RC Su, LR McKinnon, H Pymar, V Poliquin, AR Berard, A Burgener, TT Murooka
Mucosal Immunology, 2023-04-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Uropathogenic Escherichia coli infection-induced epithelial trained immunity impacts urinary tract disease outcome
Authors: SK Russell, JK Harrison, BS Olson, HJ Lee, VP O'Brien, X Xing, J Livny, L Yu, EDO Roberson, R Bomjan, C Fan, M Sha, S Estfanous, AO Amer, M Colonna, TS Stappenbec, T Wang, TJ Hannan, SJ Hultgren
Nature Microbiology, 2023-04-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Taste papilla cell differentiation requires tongue mesenchyme via ALK3-BMP signaling to regulate the production of secretory proteins
Authors: M Ishan, Z Wang, P Zhao, Y Yao, S Stice, L Wells, Y Mishina, HX Liu
bioRxiv : the preprint server for biology, 2023-04-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Non-canonical functions of SNAIL drive context-specific cancer progression
Authors: MC Paul, C Schneeweis, C Falcomatà, C Shan, D Rossmeisl, S Koutsouli, C Klement, M Zukowska, SA Widholz, M Jesinghaus, KK Heuermann, T Engleitner, B Seidler, K Sleiman, K Steiger, M Tschurtsch, B Walter, SA Weidemann, R Pietsch, A Schnieke, RM Schmid, MS Robles, G Andrieux, M Boerries, R Rad, G Schneider, D Saur
Nature Communications, 2023-03-07;14(1):1201.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells
Authors: W Zhang, Z Xu, X Hao, T He, J Li, Y Shen, K Liu, Y Gao, J Liu, D Edwards, AM Muscarella, L Wu, L Yu, L Xu, X Chen, YH Wu, IL Bado, Y Ding, S Aguirre, H Wang, Z Gugala, RL Satcher, ST Wong, XH Zhang
Cancer Discovery, 2023-02-06;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection
Authors: AT Tang, DW Buchholz, KM Szigety, B Imbiakha, S Gao, M Frankfurte, M Wang, J Yang, P Hewins, P Mericko-Is, NA Leu, S Sterling, IA Monreal, J Sahler, A August, X Zhu, KA Jurado, M Xu, EE Morrisey, SE Millar, HC Aguilar, ML Kahn
PloS Biology, 2023-02-06;21(2):e3001989.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Non-canonical functions of SNAIL drive context-specific cancer progression
Authors: MC Paul, C Schneeweis, C Falcomatà, C Shan, D Rossmeisl, S Koutsouli, C Klement, M Zukowska, SA Widholz, M Jesinghaus, KK Heuermann, T Engleitner, B Seidler, K Sleiman, K Steiger, M Tschurtsch, B Walter, SA Weidemann, R Pietsch, A Schnieke, RM Schmid, MS Robles, G Andrieux, M Boerries, R Rad, G Schneider, D Saur
Nature Communications, 2023;14(1):1201.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Multiscale profiling of protease activity in cancer
Authors: AP Amini, JD Kirkpatric, CS Wang, AM Jaeger, S Su, S Naranjo, Q Zhong, CM Cabana, T Jacks, SN Bhatia
Nature Communications, 2022-10-03;13(1):5745.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The NALCN channel regulates metastasis and nonmalignant cell dissemination
Authors: EP Rahrmann, D Shorthouse, A Jassim, LP Hu, M Ortiz, B Mahler-Ara, P Vogel, M Paez-Ribes, A Fatemi, GJ Hannon, R Iyer, JA Blundon, FC Lourenço, J Kay, RM Nazarian, BA Hall, SS Zakharenko, DJ Winton, L Zhu, RJ Gilbertson
Nature Genetics, 2022-09-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition
Authors: R Hadjisavva, O Anastasiou, PS Ioannou, M Zheltkova, PA Skourides
Oncogene, 2022-07-19;40(3):111091.
Species: Xenopus
Sample Types: Embryos
Applications: IHC -
Lymphatics act as a signaling hub to regulate intestinal stem cell activity
Authors: RE Niec, T Chu, M Schernthan, S Gur-Cohen, L Hidalgo, HA Pasolli, KA Luckett, Z Wang, SR Bhalla, F Cambuli, RP Kataru, K Ganesh, BJ Mehrara, D Pe'er, E Fuchs
Cell Stem Cell, 2022-06-20;29(7):1067-1082.e18.
Species: Human, Mouse
Sample Types: Cell Lysates, Tissue Homogenates
Applications: Western Blot -
Ex Vivo Perfusion Using a Mathematical Modeled, Controlled Gas Exchange Self-Contained Bioreactor Can Maintain a Mouse Kidney for Seven Days
Authors: N Won, J Castillo-P, X Tan, J Ford, D Heath, LI Mazilescu, M Selzner, IM Rogers
Cells, 2022-06-02;11(11):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A myosin chaperone, UNC-45A, is a novel regulator of intestinal epithelial barrier integrity and repair.
Authors: Susana L, Alexander C, Afshin K et al.
FASEB J.
-
Amniogenesis occurs in two independent waves in primates
Authors: M Rostovskay, S Andrews, W Reik, PJ Rugg-Gunn
Cell Stem Cell, 2022-04-18;29(5):744-759.e6.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Loss of Rnf31 and Vps4b sensitizes pancreatic cancer to T cell-mediated killing
Authors: N Frey, L Tortola, D Egli, S Janjuha, T Rothgangl, KF Marquart, F Ampenberge, M Kopf, G Schwank
Nature Communications, 2022-04-04;13(1):1804.
Species: Human
Sample Types: Organoids
Applications: IHC -
Suspension culture promotes serosal mesothelial development in human intestinal organoids
Authors: MM Capeling, S Huang, CJ Childs, JH Wu, YH Tsai, A Wu, N Garg, EM Holloway, N Sundaram, C Bouffi, M Helmrath, JR Spence
Cell Reports, 2022-02-15;38(7):110379.
Species: Human
Sample Types: Oraganoids
Applications: IHC -
E-Cadherin-Deficient Epithelial Cells Are Sensitive to HDAC Inhibitors
Authors: L Decourtye-, N Bougen-Zhu, T Godwin, T Brew, E Schulpen, MA Black, P Guilford
Cancers, 2021-12-30;14(1):.
Species: Mouse
Sample Types: Organoids
Applications: IHC, Western Blot -
Loss of E-Cadherin Leads to Druggable Vulnerabilities in Sphingolipid Metabolism and Vesicle Trafficking
Authors: T Brew, N Bougen-Zhu, W Mitchell, L Decourtye, E Schulpen, Y Nouri, T Godwin, P Guilford
Cancers, 2021-12-26;14(1):.
Species: Transgenic Mouse
Sample Types: Organoids
Applications: IHC -
Differentiation of mouse fetal lung alveolar progenitors in serum-free organotypic cultures
Authors: K Gkatzis, P Panza, S Peruzzo, DY Stainier
Elife, 2021-09-29;10(0):.
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Reciprocal interplay between asporin and decorin: Implications in gastric cancer prognosis
Authors: D Basak, Z Jamal, A Ghosh, PK Mondal, P Dey Talukd, S Ghosh, B Ghosh Roy, R Ghosh, A Halder, A Chowdhury, GK Dhali, BK Chattopadh, ML Saha, A Basu, S Roy, C Mukherjee, NK Biswas, U Chatterji, S Datta
PLoS ONE, 2021-08-11;16(8):e0255915.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Primary cilia-dependent lipid raft/caveolin dynamics regulate adipogenesis
Authors: D Yamakawa, D Katoh, K Kasahara, T Shiromizu, M Matsuyama, C Matsuda, Y Maeno, M Watanabe, Y Nishimura, M Inagaki
Cell Reports, 2021-03-09;34(10):108817.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro
Authors: Sawaied A, Arazi E, AbuElhija A et al.
International Journal of Molecular Sciences
-
Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis
Authors: P Lu, Y Yamaguchi, WB Fulton, S Wang, Q Zhou, H Jia, ML Kovler, AG Salazar, M Sampah, T Prindle, P Wipf, CP Sodhi, DJ Hackam
Nature Communications, 2021-02-15;12(1):1042.
Species: Human, Mouse, Porcine
Sample Types: Whole Tissue
Applications: IHC -
The Expression Levels and Cellular Localization of Pigment Epithelium Derived Factor (PEDF) in Mouse Testis: Its Possible Involvement in the Differentiation of Spermatogonial Cells
Authors: N Bagdadi, A Sawaied, A AbuMadighe, E Lunenfeld, M Huleihel
International Journal of Molecular Sciences, 2021-01-24;22(3):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mapping Development of the Human Intestinal Niche at Single-Cell Resolution
Authors: EM Holloway, M Czerwinski, YH Tsai, JH Wu, A Wu, CJ Childs, KD Walton, CW Sweet, Q Yu, I Glass, B Treutlein, JG Camp, JR Spence
Cell Stem Cell, 2020-12-04;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Three-Dimensional Human Alveolar Stem Cell Culture Models Reveal Infection Response to SARS-CoV-2
Authors: J Youk, T Kim, KV Evans, YI Jeong, Y Hur, SP Hong, JH Kim, K Yi, SY Kim, KJ Na, T Bleazard, HM Kim, M Fellows, KT Mahbubani, K Saeb-Parsy, SY Kim, YT Kim, GY Koh, BS Choi, YS Ju, JH Lee
Cell Stem Cell, 2020-10-21;27(6):905-919.e10.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure
Authors: H Hanyu, Y Yokoi, K Nakamura, T Ayabe, K Tanaka, K Uno, K Miyajima, Y Saito, K Iwatsuki, M Shimizu, M Tadaishi, K Kobayashi-
Toxins (Basel), 2020-09-24;12(10):.
Species: Mouse
Sample Types: Organoid
Applications: IHC -
Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth
Authors: Krivanek J, Soldatov RA, Kastriti ME et al.
Nature Communications
-
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dysregulation of intestinal epithelial CFTR-dependent Cl- ion transport and paracellular barrier function drives gastrointestinal symptoms of food-induced anaphylaxis in mice
Authors: A Yamani, D Wu, R Ahrens, L Waggoner, TK Noah, V Garcia-Her, C Ptaschinsk, CA Parkos, NW Lukacs, A Nusrat, SP Hogan
Mucosal Immunol, 2020-06-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CD137 Signaling Regulates Acute Colitis via RALDH2-Expressing CD11b-CD103+ DCs
Authors: J Jin, IH Jung, SH Moon, S Jeon, SJ Jeong, SK Sonn, S Seo, MN Lee, EJ Song, HY Kweon, S Kim, TK Kim, J Kim, HR Cho, JH Choi, B Kwon, GT Oh
Cell Rep, 2020-03-24;30(12):4124-4136.e5.
Species: Mouse
Sample Types: Colon Tissue
Applications: IHC -
Genome-Scale CRISPR Screening in Human Intestinal Organoids Identifies Drivers of TGF-&beta Resistance
Authors: T Ringel, N Frey, F Ringnalda, S Janjuha, S Cherkaoui, S Butz, S Srivatsa, M Pirkl, G Russo, L Villiger, G Rogler, H Clevers, N Beerenwink, N Zamboni, T Baubec, G Schwank
Cell Stem Cell, 2020-03-05;26(3):431-440.e8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis
Authors: AD Werts, WB Fulton, MR Ladd, A Saad-Eldin, YX Chen, ML Kovler, H Jia, EC Banfield, R Buck, K Goerhing, T Prindle, S Wang, Q Zhou, P Lu, Y Yamaguchi, CP Sodhi, DJ Hackam
Cell Mol Gastroenterol Hepatol, 2019-11-19;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lack of whey acidic protein four disulphide core (WFDC) 2 protease inhibitor causes neonatal death from respiratory failure in mice
Authors: K Nakajima, M Ono, U Radovi?, S Dizdarevi?, SI Tomizawa, K Kuroha, G Naganatsu, I Hoshi, R Matsunaga, T Shirakawa, T Kurosawa, Y Miyazaki, M Seki, Y Suzuki, H Koseki, M Nakamura, T Suda, K Ohbo
Dis Model Mech, 2019-11-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo
Authors: M Reed, AC Luissint, V Azcutia, S Fan, MN O'Leary, M Quiros, J Brazil, A Nusrat, CA Parkos
Nat Commun, 2019-11-01;10(1):5004.
Species: Mouse
Sample Types: Cells
Applications: IF -
Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells
Authors: C Liu, T Tate, E Batourina, ST Truschel, S Potter, M Adam, T Xiang, M Picard, M Reiley, K Schneider, M Tamargo, C Lu, X Chen, J He, H Kim, CL Mendelsohn
Nat Commun, 2019-10-09;10(1):4589.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing polycystic kidney disease
Authors: MK Yanda, B Cha, C Cebotaru, L Cebotaru
J. Biol. Chem., 2019-09-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Confocal Microscopy -
Loss of BCL9/9l suppresses Wnt driven tumourigenesis in models that recapitulate human cancer
Authors: DM Gay, RA Ridgway, M Müeller, MC Hodder, A Hedley, W Clark, JD Leach, R Jackstadt, C Nixon, DJ Huels, AD Campbell, TG Bird, OJ Sansom
Nat Commun, 2019-02-13;10(1):723.
Species: Mouse
Sample Types: Whole Tissue
Applications: Proximity Ligation Assay (PLA) -
Pancreatic Cell Fate Determination Relies on Notch Ligand Trafficking by NFIA
Authors: MA Scavuzzo, J Chmielowie, D Yang, K Wamble, LS Chaboub, L Duraine, B Tepe, SM Glasgow, BR Arenkiel, C Brou, B Deneen, M Borowiak
Cell Rep, 2018-12-26;25(13):3811-3827.e7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Host restriction of Escherichia coli recurrent urinary tract infection occurs in a bacterial strain-specific manner
Authors: VP O'Brien, DA Dorsey, TJ Hannan, SJ Hultgren
PLoS Pathog., 2018-12-13;14(12):e1007457.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model
Authors: S Flemming, AC Luissint, A Nusrat, CA Parkos
JCI Insight, 2018-10-18;3(20):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Polyploid Superficial Cells that Maintain the Urothelial Barrier Are Produced via Incomplete Cytokinesis and Endoreplication
Authors: J Wang, E Batourina, K Schneider, S Souza, T Swayne, C Liu, CD George, T Tate, H Dan, G Wiessner, Y Zhuravlev, JC Canman, IU Mysorekar, CL Mendelsohn
Cell Rep, 2018-10-09;25(2):464-477.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Desmosomal cadherin association with Tctex-1 and cortactin-Arp2/3 drives perijunctional actin polymerization to promote keratinocyte delamination
Authors: O Nekrasova, RM Harmon, JA Broussard, JL Koetsier, LM Godsel, GN Fitz, ML Gardel, KJ Green
Nat Commun, 2018-03-13;9(1):1053.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
NANOS2 acts as an intrinsic regulator of gonocytes-to-spermatogonia transition in the murine testes
Authors: HP Pui, Y Saga
Mech. Dev., 2018-01-12;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Combined mutation of Apc, Kras and Tgfbr2 effectively drives metastasis of intestinal cancer
Authors: E Sakai, M Nakayama, H Oshima, Y Kouyama, A Niida, S Fujii, A Ochiai, KI Nakayama, K Mimori, Y Suzuki, CP Hong, CY Ock, SJ Kim, M Oshima
Cancer Res., 2017-12-27;0(0):.
-
Multicolor quantitative confocal imaging cytometry
Authors: DL Coutu, KD Kokkaliari, L Kunz, T Schroeder
Nat. Methods, 2017-11-13;15(1):39-46.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic ? Cell Hyperplasia in Mice
Authors: J Kim, H Okamoto, Z Huang, G Anguiano, S Chen, Q Liu, K Cavino, Y Xin, E Na, R Hamid, J Lee, B Zambrowicz, R Unger, AJ Murphy, Y Xu, GD Yancopoulo, WH Li, J Gromada
Cell Metab., 2017-06-06;25(6):1348-1361.e8.
-
Distinct Requirements for FGFR1 and FGFR2 in Primitive Endoderm Development and Exit from Pluripotency
Authors: A Molotkov, P Mazot, JR Brewer, RM Cinalli, P Soriano
Dev. Cell, 2017-05-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection
Authors: P Treerat, O Prince, A Cruz-Lagun, M Muñoz-Torr, MA Salazar-Le, M Selman, B Fallert-Ju, TA Reinhardt, JF Alcorn, D Kaushal, J Zuñiga, J Rangel-Mor, JK Kolls, SA Khader
Mucosal Immunol, 2017-03-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Maintenance of Taste Organs Is Strictly Dependent on Epithelial Hedgehog/GLI Signaling
PLoS Genet, 2016-11-28;12(11):e1006442.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease
Nat Microbiol, 2016-10-31;2(0):16196.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Dazl is a target RNA suppressed by mammalian NANOS2 in sexually differentiating male germ cells
Authors: Y Kato, T Katsuki, H Kokubo, A Masuda, Y Saga
Nat Commun, 2016-04-13;7(0):11272.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
E-cadherin can limit the transforming properties of activating beta-catenin mutations.
Authors: Huels D, Ridgway R, Radulescu S, Leushacke M, Campbell A, Biswas S, Leedham S, Serra S, Chetty R, Moreaux G, Parry L, Matthews J, Song F, Hedley A, Kalna G, Ceteci F, Reed K, Meniel V, Maguire A, Doyle B, Soderberg O, Barker N, Watson A, Larue L, Clarke A, Sansom O
EMBO J, 2015-08-03;34(18):2321-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion.
Authors: Ihermann-Hella A, Lume M, Miinalainen I, Pirttiniemi A, Gui Y, Peranen J, Charron J, Saarma M, Costantini F, Kuure S
PLoS Genet, 2014-03-06;10(3):e1004193.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
AKT3 regulates ErbB2, ErbB3 and estrogen receptor alpha expression and contributes to endocrine therapy resistance of ErbB2(+) breast tumor cells from Balb-neuT mice.
Authors: Grabinski N, Mollmann K, Milde-Langosch K, Muller V, Schumacher U, Brandt B, Pantel K, Jucker M
Cell Signal, 2014-01-24;26(5):1021-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Annexin A2 mediates secretion of collagen VI, pulmonary elasticity and apoptosis of bronchial epithelial cells.
Authors: Dassah M, Almeida D, Hahn R, Bonaldo P, Worgall S, Hajjar K
J Cell Sci, 2013-12-19;127(0):828-44.
Species: Mouse
Sample Types: Whole Tissue
Applications: Neutralization -
Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules.
Authors: Miyauchi, Eiji, Ogita, Tasuku, Miyamoto, Junki, Kawamoto, Seiji, Morita, Hidetosh, Ohno, Hiroshi, Suzuki, Takuya, Tanabe, Soichi
PLoS ONE, 2013-11-08;8(11):e79735.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
MMP20 modulates cadherin expression in ameloblasts as enamel develops.
Authors: Guan X, Bartlett J
2013-09-25;92(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury.
Authors: Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield J
J Am Soc Nephrol, 2013-03-14;24(4):559-72.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis.
Authors: Neal M, Sodhi C, Dyer M, Craig B, Good M, Jia H, Yazji I, Afrazi A, Richardson W, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca M, Ozolek J, Hackam D
J Immunol, 2013-03-01;190(7):3541-51.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
N-cadherin+ HSCs in fetal liver exhibit higher long-term bone marrow reconstitution activity than N-cadherin- HSCs.
Biochem Biophys Res Commun, 2012-10-22;428(3):354-9.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC-Fr -
Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli.
Authors: Betterman K, Paquet-Fifield S, Asselin-Labat M, Visvader J, Butler L, Stacker S, Achen M, Harvey N
Am J Pathol, 2012-10-11;181(6):2225-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Epithelial cell-intrinsic notch signaling plays an essential role in the maintenance of gut immune homeostasis.
Authors: Obata Y, Takahashi D, Ebisawa M, Kakiguchi K, Yonemura S, Jinnohara T, Kanaya T, Fujimura Y, Ohmae M, Hase K, Ohno H
J. Immunol., 2012-01-25;188(5):2427-36.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Noninvasive assessment of antenatal hydronephrosis in mice reveals a critical role for Robo2 in maintaining anti-reflux mechanism.
Authors: Wang H, Li Q, Liu J
PLoS ONE, 2011-09-20;6(9):e24763.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors.
Authors: Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM
Cancer Res., 2006-02-01;66(3):1434-45.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons.
Authors: Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR
Development, 2005-07-27;132(17):3847-57.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice.
Authors: Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA
Kidney Int., 2005-06-01;67(6):2221-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse E-Cadherin Antibody
Average Rating: 4.6 (Based on 10 Reviews)
Have you used Human/Mouse E-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: