Human/Primate VEGF Antibody Summary
Ala27-Arg191
Accession # NP_001165097.1
Applications
Human/Primate VEGF Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
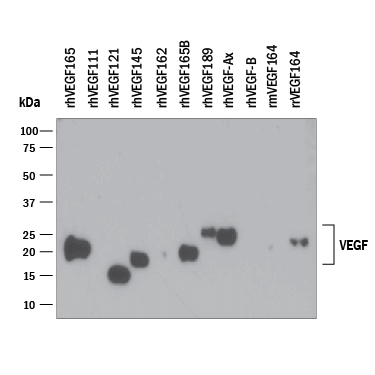
Detection of Recombinant Human VEGF by Western Blot. Western blot shows 25 ng of Recombinant Human VEGF165(Catalog # 293-VE), Recombinant Human VEGF111(Catalog # 5336-VE), Recombinant Human VEGF121, aa 207-327 (Catalog # 4644-VS), Recombinant Human VEGF145(aa 27-171) (Catalog # 7626-VE), Recombinant Human VEGF162(Catalog # 2347-VE), Recombinant Human VEGF165b(Catalog # 3045-VE), Recombinant Human VEGF189(aa 27-215) (Catalog # 8147-VE), Recombinant Human VEGF165Extended Isoform (Catalog # 9018-VE), Recombinant Human VEGF-B167(Catalog # 751-VE), Recombinant Mouse VEGF164(Catalog # 493-MV), and Recombinant Rat VEGF164(Catalog # 564-RV). PVDF Membrane was probed with 0.1 µg/mL of Mouse Anti-Human/Primate VEGF Monoclonal Antibody (Catalog # MAB293) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF007). A specific band was detected for VEGF at approximately 15-25 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 3.
 View Larger
View Larger
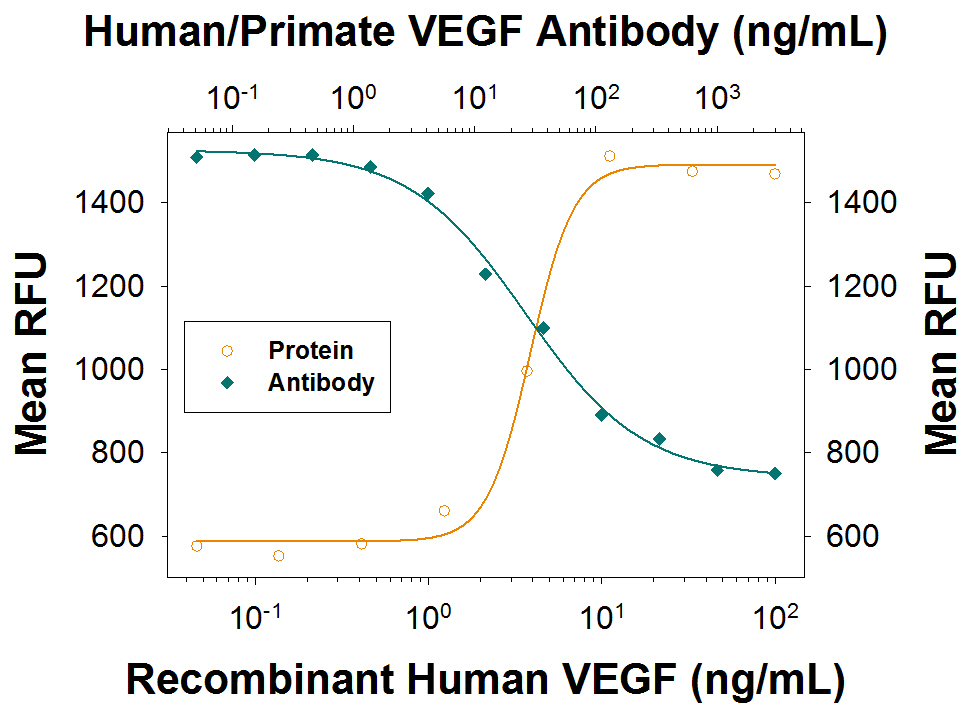
Cell Proliferation Induced by VEGF165and Neutralization by Human VEGF Antibody. Recombinant Human VEGF165(Catalog # 293-VE) stimulates proliferation in HUVEC human umbilical vein endothelial cells in a dose-dependent manner (orange line) as measured by Resazurin (Catalog # AR002). Proliferation elicited by Recombinant Human VEGF165(10 ng/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-Human/Primate VEGF Monoclonal Antibody (Catalog # MAB293). The ND50 is typically 10-60 ng/mL.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VEGF
Vascular Endothelial Growth Factor (VEGF or VEGF-A), also known as Vascular Permeability Factor (VPF), is a potent mediator of both angiogenesis and vasculogenesis in the fetus and adult. It is a member of the PDGF family that is characterized by the presence of eight conserved cysteine residues and a cystine knot structure. VEGF165 appears to be the most abundant and potent isoform, followed by VEGF121 and VEGF189. Human VEGF165 is an approximately 44 kDa molecular weight homodimer sharing 88% aa sequence identity with corresponding regions of mouse and rat, 96% with porcine, 95% with canine, and 93% with feline, equine and bovine VEGF, respectively. VEGF binds the type I transmembrane receptor tyrosine kinases VEGF R1 (also called Flt-1) and VEGF R2 (Flk-1/KDR) on endothelial cells. Although VEGF affinity is highest for binding to VEGF R1, VEGF R2 appears to be the primary mediator of VEGF angiogenic activity. VEGF165 binds the Semaphorin receptor, Neuropilin-1 and promotes complex formation with VEGF R2. VEGF is required during embryogenesis and functions to regulate the proliferation, migration, and survival of endothelial cells. In adults, VEGF functions mainly in wound healing and the female reproductive cycle. Pathologically, it is involved in tumor angiogenesis and vascular leakage. Circulating VEGF levels correlate with disease activity in autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus. VEGF is induced by hypoxia and cytokines such as IL-1, IL-6, IL-8, Oncostatin M (OSM) and TNF-alpha.
Product Datasheets
Citations for Human/Primate VEGF Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
62
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Fibrocytes boost tumor-supportive phenotypic switches in the lung cancer niche via the endothelin system
Authors: A Weigert, X Zheng, A Nenzel, K Turkowski, S Günther, E Strack, E Sirait-Fis, E Elwakeel, IM Kur, VS Nikam, C Valasaraja, H Winter, A Wissgott, R Voswinkel, F Grimminger, B Brüne, W Seeger, SS Pullamsett, R Savai
Nature Communications, 2022-10-14;13(1):6078.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Effects of Human Deciduous Dental Pulp-Derived Mesenchymal Stem Cell-Derived Conditioned Medium on the Metabolism of HUVECs, Osteoblasts, and BMSCs
Authors: R Kunimatsu, T Hiraki, K Rikitake, K Nakajima, NAR Putranti, T Abe, K Ando, A Nakatani, S Sakata, K Tanimoto
Cells, 2022-10-14;11(20):.
Species: Human
Sample Types: Whole Cells
Applications: ELISA Development, Neutralization -
Inhibition of the VEGF signaling pathway attenuates tumor?associated macrophage activity in liver cancer
Authors: S Okikawa, Y Morine, Y Saito, S Yamada, K Tokuda, H Teraoku, K Miyazaki, S Yamashita, T Ikemoto, S Imura, M Shimada
Oncology reports, 2022-02-16;47(4):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Tissue Inhibitor of Metalloproteinase-3 Ameliorates Diabetes-Induced Retinal Inflammation
Authors: AM Abu El-Asr, A Ahmad, MI Nawaz, MM Siddiquei, A De Zutter, L Vanbrabant, PW Gikandi, G Opdenakker, S Struyf
Frontiers in Physiology, 2022-01-10;12(0):807747.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
CD146/Soluble CD146 Pathway Is a Novel Biomarker of Angiogenesis and Inflammation in Proliferative Diabetic Retinopathy
Authors: AM Abu El-Asr, MI Nawaz, A Ahmad, MM Siddiquei, E Allegaert, PW Gikandi, G De Hertogh, G Opdenakker
Investigative Ophthalmology & Visual Science, 2021-07-01;62(9):32.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
3D iPSC modeling of the retinal pigment epithelium-choriocapillaris complex identifies factors involved in the pathology of macular degeneration
Authors: KV Manian, CA Galloway, S Dalvi, AA Emanuel, JA Mereness, W Black, L Winschel, C Soto, Y Li, Y Song, W DeMaria, A Kumar, I Slukvin, MP Schwartz, WL Murphy, B Anand-Apte, M Chung, DSW Benoit, R Singh
Cell Stem Cell, 2021-03-29;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation
Authors: ZY Liao, IC Liang, HJ Li, CC Wu, HM Lo, DC Chang, CF Hung
Int J Mol Sci, 2020-08-02;21(15):.
Species: Primate, Rhesus Macaque
Sample Types: Cell Lysates
Applications: Western Blot -
Snail promotes the generation of vascular endothelium by breast cancer cells
Authors: Z Chang, Y Zhang, J Liu, Y Zheng, H Li, Y Kong, P Li, H Peng, Y Shi, B Cao, F Ran, Y Chen, Y Song, Q Ye, L Ding
Cell Death Dis, 2020-06-15;11(6):457.
Species: Human
Sample Types: Transduced Whole Cells
Applications: Neutralization -
Migration Inhibitory Factor in Conditioned Medium from Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Stimulates Hair Growth
Authors: HA Oh, J Kwak, BJ Kim, HJ Jin, WS Park, SJ Choi, W Oh, S Um
Cells, 2020-05-28;9(6):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesis in rats fed a high-fat diet
Authors: DJ Perdicaro, C Rodriguez, J Gambarte T, RM Miatello, PI Oteiza, MA Vazquez Pr
J. Nutr. Biochem., 2020-02-04;79(0):108352.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Contribution of the VEGF system to the follicular persistence associated with bovine cystic ovaries
Authors: AF Stassi, F Gasser, MML Velázquez, EM Belotti, NC Gareis, F Rey, HH Ortega, NR Salvetti, ME Baravalle
Theriogenology, 2019-07-03;138(0):52-65.
Species: Bovine, Mouse
Sample Types: Cell Lysates, Tissue Homogenates, Whole Tissue
Applications: IHC, IHC-P, Western Blot -
TRF2 positively regulates SULF2 expression increasing VEGF-A release and activity in tumor microenvironment
Authors: P Zizza, R Dinami, M Porru, C Cingolani, E Salvati, A Rizzo, C D'Angelo, E Petti, CA Amoreo, M Mottolese, I Sperduti, A Chambery, R Russo, P Ostano, G Chiorino, G Blandino, A Sacconi, J Cherfils-V, C Leonetti, E Gilson, A Biroccio
Nucleic Acids Res., 2019-04-23;0(0):.
Species: Human, Mouse
Sample Types: In Vivo, Whole Cells
Applications: Neutralization -
TSP-1 is downregulated and inversely correlates with miR-449c expression in Cushing's disease
Authors: J Ren, C Gu, Y Yang, J Xue, Y Sun, F Jian, D Chen, L Bian, Q Sun
J. Cell. Mol. Med., 2019-04-23;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
A Subset of Paracrine Factors as Efficient Biomarkers for Predicting Vascular Regenerative Efficacy of Mesenchymal Stromal/Stem Cells
Authors: HK Kim, SG Lee, SW Lee, BJ Oh, JH Kim, JA Kim, G Lee, JD Jang, YA Joe
Stem Cells, 2018-10-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface
Authors: MKS Tang, PYK Yue, PP Ip, RL Huang, HC Lai, ANY Cheung, KY Tse, HYS Ngan, AST Wong
Nat Commun, 2018-06-11;9(1):2270.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Grape pomace extract induced beige cells in white adipose tissue from rats and in 3T3-L1 adipocytes
Authors: C Rodriguez, DJ Perdicaro, MS Landa, A Fontana, A Antoniolli, RM Miatello, PI Oteiza, MA Vazquez Pr
J. Nutr. Biochem., 2018-03-10;56(0):224-233.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Targeting angiogenesis in multiple myeloma by the VEGF and HGF blocking DARPin� protein MP0250: a preclinical study
Authors: L Rao, K De Veirman, D Giannico, I Saltarella, V Desantis, MA Frassanito, AG Solimando, D Ribatti, M Prete, A Harstrick, U Fiedler, H De Raeve, V Racanelli, K Vanderkerk, A Vacca
Oncotarget, 2018-01-30;9(17):13366-13381.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Investigating Colorimetric Protein Array Assay Schemes for Detection of Recurrence of Bladder Cancer
Authors: S Gogalic, U Sauer, S Doppler, C Preininger
Biosensors (Basel), 2018-01-24;8(1):.
Species: Human
Sample Types: Urine
Applications: Array Development -
Application of an acellular dermal matrix to a rabbit model of oral mucosal defects
Authors: X Xu, N Cui, E Wang
Exp Ther Med, 2018-01-05;15(3):2450-2456.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Predictive Value of Clinical Findings and Plasma Biomarkers after Fourteen Days of Prednisone Treatment for Acute Graft-versus-host Disease.
Authors: McDonald G, Tabellini L, Storer B, Martin P, Lawler R, Rosinski S, Schoch H, Hansen J
Biol Blood Marrow Transplant, 2017-05-03;23(8):1257-1263.
Species: Human
Sample Types: Plasma
-
HIF1? regulates single differentiated glioma cell dedifferentiation to stem-like cell phenotypes with high tumorigenic potential under hypoxia
Authors: P Wang, C Lan, S Xiong, X Zhao, Y Shan, R Hu, W Wan, S Yu, B Liao, G Li, J Wang, D Zou, B Chen, H Feng, N Wu
Oncotarget, 2017-04-25;8(17):28074-28092.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Vessel morphometric parameters-correlation with histologic grade and VEGF expression in oligodendroglioma
Authors: LB Strickland, S Brem, AM Rojiani, MV Rojiani
Am J Cancer Res, 2017-04-01;7(4):973-981.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells
Authors: W Katagiri, T Kawai, M Osugi, Y Sugimura-W, K Sakaguchi, T Kojima, T Kobayashi
Maxillofac Plast Reconstr Surg, 2017-03-25;39(1):8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Development of a surrogate potency assay to determine the angiogenic activity of Stempeucel�, a pooled, ex-vivo expanded, allogeneic human bone marrow mesenchymal stromal cell product
Authors: C Thej, B Ramadasse, A Walvekar, AS Majumdar, S Balasubram
Stem Cell Res Ther, 2017-02-28;8(1):47.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Transendothelial migration of human umbilical mesenchymal stem cells across uterine endothelial monolayers: Junctional dynamics and putative mechanisms
Authors: Lopa Leach
Placenta, 2016-10-21;48(0):87-98.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Neutralization -
The Proton-Sensing G-Protein Coupled Receptor GPR4 Promotes Angiogenesis in Head and Neck Cancer
Authors: Z Jing, H Xu, X Chen, Q Zhong, J Huang, Y Zhang, W Guo, Z Yang, S Ding, P Chen, Z Huang
PLoS ONE, 2016-04-14;11(4):e0152789.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors
Authors: D Grun, G Adhikary, RL Eckert
Oncogene, 2016-01-25;35(33):4379-87.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Interleukin-1 Receptor Type 2 Acts with c-Fos to Enhance the Expression of Interleukin-6 and Vascular Endothelial Growth Factor A in Colon Cancer Cells and Induce Angiogenesis.
Authors: Mar A, Chu C, Lee H, Chien C, Cheng J, Yang S, Jiang J, Lee T
J Biol Chem, 2015-07-24;290(36):22212-24.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Pericytes contribute to the disruption of the cerebral endothelial barrier via increasing VEGF expression: implications for stroke.
Authors: Bai Y, Zhu X, Chao J, Zhang Y, Qian C, Li P, Liu D, Han B, Zhao L, Zhang J, Buch S, Teng G, Hu G, Yao H
PLoS ONE, 2015-04-17;10(4):e0124362.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced beta-catenin/Tcf-Lef dependent transcription.
Authors: Fernandez J, Rodriguez D, Valenzuela M, Calderon C, Urzua U, Munroe D, Rosas C, Lemus D, Diaz N, Wright M, Leyton L, Tapia J, Quest A
Mol Cancer, 2014-09-09;13(0):209.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis.
Authors: Lee E, Fertig E, Jin K, Sukumar S, Pandey N, Popel A
Nat Commun, 2014-09-02;5(0):4715.
Species: Human
Sample Types: In Vivo, Whole Tissue
Applications: IHC, Neutralization -
Interleukin 4, interleukin 6 and osteopontin-serological markers of head and neck malignancy in primary diagnostics: A pilot study.
Authors: Aderhold C, Grobschmidt G, Sauter A, Faber A, Hormann K, Schultz J
Oncol Lett, 2014-07-04;8(3):1112-1118.
Species: Human
Sample Types: Serum
Applications: Bioassay -
Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors.
Authors: Etulain J, Negrotto S, Tribulatti M, Croci D, Carabelli J, Campetella O, Rabinovich G, Schattner M
PLoS ONE, 2014-04-30;9(4):e96402.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Enhanced efficacy of combination therapy with adenoassociated virus-delivered pigment epithelium-derived factor and cisplatin in a mouse model of Lewis lung carcinoma.
Authors: He S, Wu Q, Gong C, Luo S, Zhang S, Li M, Lu L, Wei Y, Yang L
Mol Med Rep, 2014-04-04;9(6):2069-76.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Hematopoietic stem cell cytokines and fibroblast growth factor-2 stimulate human endothelial cell-pericyte tube co-assembly in 3D fibrin matrices under serum-free defined conditions.
Authors: Smith A, Bowers S, Stratman A, Davis G
PLoS ONE, 2013-12-31;8(12):e85147.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Markers of T cell infiltration and function associate with favorable outcome in vascularized high-grade serous ovarian carcinoma.
Authors: Townsend, Katelin, Spowart, Jaeline, Huwait, Hassan, Eshragh, Sima, West, Nathan R, Elrick, Mary A, Kalloger, Steve E, Anglesio, Michael, Watson, Peter H, Huntsman, David G, Lum, Julian J
PLoS ONE, 2013-12-23;8(12):e82406.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Heat shock protein 27 attenuates neointima formation and accelerates reendothelialization after arterial injury and stent implantation: importance of vascular endothelial growth factor up-regulation.
Authors: Ma X, Hibbert B, McNulty M, Hu T, Zhao X, Ramirez F, Simard T, de Belleroche J, O'Brien E
FASEB J, 2013-10-18;28(2):594-602.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha.
Authors: Tan J, Prosser H, Vanags L, Monger S, Ng M, Bursill C
FASEB J, 2013-09-10;28(1):206-17.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The transcription factor E2F1 and the SR protein SC35 control the ratio of pro-angiogenic versus antiangiogenic isoforms of vascular endothelial growth factor-A to inhibit neovascularization in vivo.
Authors: Merdzhanova G, Gout S, Keramidas M, Edmond V, Coll JL, Brambilla C, Brambilla E, Gazzeri S, Eymin B
Oncogene, 2010-07-19;29(39):5392-403.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
VEGF in the lung: a role for novel isoforms.
Authors: Varet J, Varet, Julia, Douglas SK, Douglas, Samantha, Gilmartin L, Gilmartin, Laura, Medford, Andrew R, Bates, David O, Harper, Steven J, Millar, Ann B
Am J Physiol Lung Cell Mol Physiol, 2010-03-12;298(6):L768-74.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth.
Authors: Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D, Bready JV, Oliner JD, McDonald DM
Cancer Res., 2010-03-02;70(6):2213-23.
Species: Human
Sample Types: In Vivo
Applications: Neutralization -
A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-kappa B pathway.
Authors: Zhu W, Ou Y, Li Y, Xiao R, Shu M, Zhou Y, Xie J, He S, Qiu P, Yan G
Mol. Pharmacol., 2009-01-21;75(4):812-9.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Development and validation of sandwich ELISA microarrays with minimal assay interference.
Authors: Gonzalez RM, Seurynck-Servoss SL, Crowley SA
J. Proteome Res., 2008-04-19;7(6):2406-14.
Species: Human
Sample Types: Serum
Applications: ELISA Microarray Development -
FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities.
Authors: Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, Czech T, Marosi C, Buchroithner J, Pichler J, Silye R, Mohr T, Holzmann K, Grasl-Kraupp B, Marian B, Grusch M, Fischer J, Micksche M, Berger W
Oncogene, 2008-03-24;27(30):4180-90.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Ultrasensitive flow-based immunoassays using single-molecule counting.
Authors: Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, Goix P
Clin. Chem., 2007-09-21;53(11):1990-5.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Vascular endothelial growth factor can signal through platelet-derived growth factor receptors.
Authors: Ball SG, Shuttleworth CA, Kielty CM
J. Cell Biol., 2007-04-30;177(3):489-500.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts.
Authors: Hatfield K, Ryningen A, Corbascio M, Bruserud O
Int. J. Cancer, 2006-11-15;119(10):2313-21.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential.
Authors: Cianfarani F, Zambruno G, Brogelli L, Sera F, Lacal PM, Pesce M, Capogrossi MC, Failla CM, Napolitano M, Odorisio T
Am. J. Pathol., 2006-10-01;169(4):1167-82.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Vascular endothelial growth factor-A induces plaque expansion in ApoE knock-out mice by promoting de novo leukocyte recruitment.
Authors: Lucerna M, Zernecke A, de Nooijer R, de Jager SC, Bot I, van der Lans C, Kholova I, Liehn EA, van Berkel TJ, Yla-Herttuala S, Weber C, Biessen EA
Blood, 2006-09-21;109(1):122-9.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice.
Authors: Rofstad EK, Mathiesen B, Kindem K, Galappathi K
Cancer Res., 2006-07-01;66(13):6699-707.
Species: Complex Species Category
Sample Types: In Vivo
Applications: Neutralization -
The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term.
Authors: Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ
Clin. Sci., 2006-05-01;110(5):575-85.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma.
Authors: Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, Walters EH
Am. J. Respir. Crit. Care Med., 2006-03-09;173(11):1201-7.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis.
Authors: Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H
J. Immunol., 2005-11-01;175(9):6177-89.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Functional and structural remodeling of the myocardial microvasculature in early experimental hypertension.
Authors: Rodriguez-Porcel M, Zhu XY, Chade AR, Amores-Arriaga B, Caplice NM, Ritman EL, Lerman A, Lerman LO
Am. J. Physiol. Heart Circ. Physiol., 2005-10-07;290(3):H978-84.
Species: Porcine
Sample Types: Whole Tissue
Applications: IHC-Fr -
Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas.
Authors: Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K
Cancer Res., 2005-08-15;65(16):7241-8.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Evaluating hypoxia-inducible factor-1alpha as a cancer therapeutic target via inducible RNA interference in vivo.
Authors: Li L, Lin X, Staver M, Shoemaker A, Semizarov D, Fesik SW, Shen Y
Cancer Res., 2005-08-15;65(16):7249-58.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors.
Authors: Tamaki M, Saito R, Ogawa O, Yoshimura N, Ueda T
J. Urol., 2004-09-01;172(3):945-8.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Plasma angiopoietin-1, angiopoietin-2 and Tie-2 in breast and prostate cancer: a comparison with VEGF and Flt-1.
Authors: Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GY
Eur. J. Clin. Invest., 2003-10-01;33(10):883-90.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Expression of the CD44v2-10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3.
Authors: Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC
Cancer Res., 2003-02-15;63(4):887-92.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome.
Authors: Zhou Y, 2019, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ
2002-04-01;160(4):1405-23.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Hepatocyte growth factor and vascular endothelial growth factor expression in pig-to-primate xenotransplanted hearts.
Authors: Takayama K, Suzuki J, Kawauchi M, Tsukioka K, Wada Y, Zhang T, Endoh M, Takamoto S, Amano J, Isobe M
Transplant. Proc., 2000-08-01;32(5):987-9.
Species: Porcine
Sample Types: Whole Tissue
Applications: IHC-P -
Hypoxia-induced expression of VEGF is reversible in myocardial vascular smooth muscle cells.
Authors: Gu JW, Adair TH
Am. J. Physiol., 1997-08-01;273(2):H628-33.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization
FAQs
-
What are the differences between MAB293 and MAB293R?
MAB293 is produced by culturing a hybridoma cell line, whereas MAB293R is produced via recombinant DNA technology, based on the known, proprietary sequence of MAB293. The benefit of recombinant antibodies is the ability to offer a more consistent antibody, with an indefinite supply. As far as research applications, both products will work the same.
Reviews for Human/Primate VEGF Antibody
Average Rating: 4.8 (Based on 8 Reviews)
Have you used Human/Primate VEGF Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
IF of HUVEC cells with exogenous VEGF internalized (red) and colocalizing with VEGF receptor (green)
We used this VEGF-A neutralizing antibody (10 ng/mL) as part of an antibody cocktail containing several other antibodies to neutralize VEGF-A present in the Conditioned Medium (CM) from BT549 Cells treated with Angiotensin II CM (+/- VEGF-A Neutralizing ab Cocktail) was used to further look at Endothelial (HUVEC) Cell migration using Incucyte Chemotaxis Assay.
Cancer Res; 78(5) March 1, 2018 (Fig 6F)
After biotinylation, used as a capture reagent in MSD assay to detect ocular VEGF level in a swine model of laser-induced choroidal neovascularization. A standard curve with pig VEGF from Bio-Rad (Cat# PPP030) is shown (2-8,000 pg/ml).











