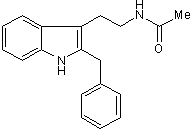
Luzindole
Chemical Name: N-Acetyl-2-benzyltryptamine
Purity: ≥98%
Biological Activity
Luzindole is a melatonin antagonist.Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Background References
-
Melatonin receptors: are there multiple subtypes?
Dubocovich
TiPS, 1995;16:50 -
Luzindole (N-0774): a novel melatonin receptor antagonist.
Dubocovich
J.Pharmacol.Exp.Ther., 1988;246:902 -
Anti-depressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in mouse behavioural despair test.
Dubocovich et al.
Eur.J.Pharmacol., 1990;182:313
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for Luzindole
The citations listed below are publications that use Tocris products. Selected citations for Luzindole include:
16 Citations: Showing 1 - 10
-
Homeostatic Plasticity Mediated by Rod-Cone Gap Junction Coupling in Retinal Degenerative Dystrophic RCS Rats.
Authors: Hou Et al.
Front Cell Neurosci 2017;11:98
-
Distinct roles of N-acetyl and 5-methoxy groups in the antiproliferative and neuroprotective effects of melatonin
Authors: Letra-Vilela Et al.
Molecular and Cellular Endocrinology 2016;434:238
-
Amyloid β peptide directly impairs pineal gland melatonin synthesis and melatonin receptor signaling through the ERK pathway.
Authors: Cecon Et al.
FASEB J 2015;29:2566
-
The melatonin agonist rame. induces duration-dependent clock gene expression through cAMP signaling in pancreatic INS-1 β-cells.
Authors: Nishiyama and Hirai
PLoS One 2014;9:e102073
-
Antinociceptive effects of novel melatonin receptor agonists in mouse models of abdominal pain.
Authors: Chen Et al.
J Clin Invest 2014;20:1298
-
Melatonin MT1 and MT2 receptors display different molecular pharmacologies only in the G-protein coupled state.
Authors: Legros Et al.
Br J Pharmacol 2014;171:186
-
NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene.
Authors: Muxel Et al.
Endocrinology 2013;7:e52010
-
Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function.
Authors: Baba Et al.
Sci Signal 2013;6:ra89
-
Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats.
Authors: Faria Et al.
Am J Physiol Endocrinol Metab 2013;305:E230
-
MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries.
Authors: Tunstall Et al.
J Neuroinflammation 2011;336:127
-
Melatonin inhibits tachykinin NK2 receptor-triggered 5-HT release from guinea pig isolated colonic mucosa.
Authors: Kojima Et al.
Br J Pharmacol 2011;162:1179
-
Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process.
Authors: Mickle Et al.
Pain 2010;149:555
-
Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro.
Authors: Silva Et al.
Br J Pharmacol 2007;151:195
-
Identification of WIN55212-3 as a competitive neutral antagonist of the human cannabinoid CB2 receptor.
Authors: Savinainen Et al.
Br J Pharmacol 2005;145:636
-
Cyclical regulation of GnRH gene expression in GT1-7 GnRH-Secr.g neurons by melatonin.
Authors: Roy Et al.
World J Gastroenterol 2001;142:4711
-
Peripheral melatonin mediates neural stimulation of duodenal mucosal bicarbonate secretion.
Authors: Sjöblom Et al.
J Pharmacol Exp Ther 2001;108:625
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for Luzindole
There are currently no reviews for this product. Be the first to review Luzindole and earn rewards!
Have you used Luzindole?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image