Mouse LYVE-1 Antibody Summary
Ala24-Thr234
Accession # Q8BHC0
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
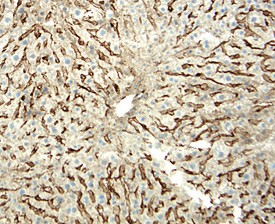
LYVE‑1 in Mouse Liver. LYVE-1 was detected in perfusion fixed frozen sections of mouse liver using 15 µg/mL Goat Anti-Mouse LYVE-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2125) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). Specific labeling was localized to the cytoplasm of endothelial cells in sinusoids. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
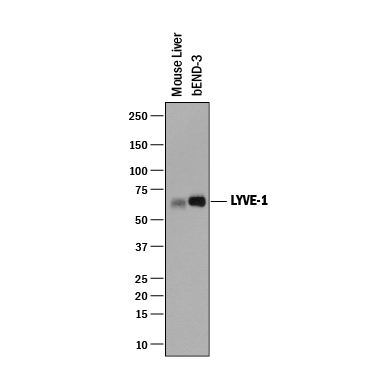
Detection of Mouse LYVE‑1 by Western Blot. Western blot shows lysates of mouse liver tissue and bEnd.3 mouse brain endothelial cell line. PVDF membrane was probed with 0.25 µg/mL of Goat Anti-Mouse LYVE-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2125) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF019). A specific band was detected for LYVE-1 at approximately 60-65 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: LYVE-1
Lymphatic vessel endothelial hyaluronan (HA) receptor-1 (LYVE-1) is a recently identified receptor of HA, a linear high molecular weight polymer composed of alternating units of D-glucuronic acid and N-acetyl-D-glucosamine. HA is found in the extracellular matrix of most animal tissues and in body fluids. It modulates cell behavior and functions during tissue remodeling, development, homeostasis, and disease. The turnover of HA (several grams/day in humans) occurs primarily in the lymphatics and liver, the two major clearance systems that catabolize approximately 85% and 15% of HA, respectively. LYVE-1 shares 41% homology with the other known HA receptor, CD44. The homology between the two proteins increases to 61% within the HA binding domain. The HA binding domain, known as the link module, is a common structural motif found in other HA binding proteins such as link protein, aggrecan and versican. Human and mouse LYVE-1 share 69% amino acid sequence identity.
LYVE-1 is primarily expressed on both the luminal and abluminal surfaces of lymphatic vessels. In addition, LYVE-1 is also present in normal hepatic blood sinusoidal endothelial cells. LYVE-1 mediates the endocytosis of HA and may transport HA from tissue to lymph by transcytosis, delivering HA to lymphatic capillaries for removal and degradation in the regional lymph nodes. Because of its restricted expression patterns, LYVE-1, along with other lymphatic proteins such as VEGF R3, podoplanin and the homeobox protein propero-related (Prox-1), constitute a set of markers useful for distinguishing between lymphatic and blood microvasculature.
Product Datasheets
Citations for Mouse LYVE-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
81
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Lipid-siRNA conjugate accesses a perivascular transport mechanism and achieves widespread and durable knockdown in the central nervous system
Authors: Sorets, AG;Schwensen, KR;Francini, N;Kjar, A;Abdulrahman, AM;Shostak, A;Katdare, KA;Schoch, KM;Cowell, RP;Park, JC;Ligocki, AP;Ford, WT;Ventura-Antunes, L;Hoogenboezem, EN;Prusky, A;Castleberry, M;Michell, DL;Miller, TM;Vickers, KC;Schrag, MS;Duvall, CL;Lippmann, ES;
bioRxiv : the preprint server for biology
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
First Application of a Mixed Porcine-Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure
Authors: Felgendreff, P;Hosseiniasl, SM;Minshew, A;Amiot, BP;Wilken, S;Ahmadzada, B;Huebert, RC;Sakrikar, NJ;Engles, NG;Halsten, P;Mariakis, K;Barry, J;Riesgraf, S;Fecteau, C;Ross, JJ;Nyberg, SL;
Biomedicines
Species: Porcine, Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Liver Sinusoidal Endothelial Cells Contribute to Portal Hypertension Through Collagen Type IV-Driven Sinusoidal Remodeling
Authors: Gan, C;Yaqoob, U;Lu, J;Xie, M;Anwar, AA;Jalan-Sakrikar, N;Jerez, S;Sehrawat, T;Navarro-Corcuera, A;Kostallari, E;Habash, NW;Cao, S;Shah, VH;
JCI insight
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Primary Cilium in Neural Crest Cells Crucial for Anterior Segment Development and Corneal Avascularity
Authors: Seo, S;Sonn, SK;Kweon, HY;Jin, J;Kume, T;Ko, JY;Park, JH;Oh, GT;
Investigative ophthalmology & visual science
Species: Viral
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Endothelial Erg Regulates Expression of Pulmonary Lymphatic Junctional and Inflammation Genes in Mouse Lungs Impacting Lymphatic Transport
Authors: Chakraborty, A;Kim, A;AlAbdullatif, S;Campbell, JD;Alekseyev, YO;Kaplan, U;Dambal, V;Ligresti, G;Trojanowska, M;
Research square
Species: Murine polyomavirus strain A3, Human hepegivirus
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-?
Authors: Zamora, A;Nougué, M;Verdu, L;Balzan, E;Draia-Nicolau, T;Benuzzi, E;Pujol, F;Baillif, V;Lacazette, E;Morfoisse, F;Galitzky, J;Bouloumié, A;Dubourdeau, M;Chaput, B;Fazilleau, N;Malloizel-Delaunay, J;Bura-Rivière, A;Prats, AC;Garmy-Susini, B;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IF/IHC -
Apelin-VEGF-C mRNA delivery as therapeutic for the treatment of secondary lymphedema
Authors: Creff, J;Lamaa, A;Benuzzi, E;Balzan, E;Pujol, F;Draia-Nicolau, T;Nougué, M;Verdu, L;Morfoisse, F;Lacazette, E;Valet, P;Chaput, B;Gross, F;Gayon, R;Bouillé, P;Malloizel-Delaunay, J;Bura-Rivière, A;Prats, AC;Garmy-Susini, B;
EMBO molecular medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Visualization of Organ-Specific Lymphatic Growth: An Efficient Approach to Labeling Molecular Markers in Cleared Tissues
Authors: C Christ, Z Jakus
International Journal of Molecular Sciences, 2023-03-07;24(6):.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
VLA-4 suppression by senescence signals regulates meningeal immunity and leptomeningeal metastasis
Authors: J Li, D Huang, B Lei, J Huang, L Yang, M Nie, S Su, Q Zhao, Y Wang
Elife, 2022-12-09;11(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ablation of lysophosphatidic acid receptor 1 attenuates hypertrophic cardiomyopathy in a mouse model
Authors: A Axelsson R, H Wakimoto, DM DeLaughter, D Reichart, J Gorham, DA Conner, M Lun, CK Probst, N Sakai, RS Knipe, SB Montesi, B Shea, LP Adam, LA Leinwand, W Wan, ES Choi, EL Lindberg, G Patone, M Noseda, N Hübner, CE Seidman, AM Tager, JG Seidman, CY Ho
Proceedings of the National Academy of Sciences of the United States of America, 2022-07-05;119(28):e2204174119.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Orphan G-Protein Coupled Receptor GPRC5B Is Critical for Lymphatic Development
Authors: W Xu, NP Nelson-Man, L Bálint, HB Kwon, RB Davis, DCM Dy, JM Dunleavey, B St Croix, KM Caron
International Journal of Molecular Sciences, 2022-05-20;23(10):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lipid droplet degradation by autophagy connects mitochondria metabolism to Prox1-driven expression of lymphatic genes and lymphangiogenesis
Authors: O Meçe, D Houbaert, ML Sassano, T Durré, H Maes, M Schaaf, S More, M Ganne, M García-Cab, M Borri, J Verhoeven, M Agrawal, K Jacobs, G Bergers, S Blacher, B Ghesquière, M Dewerchin, JV Swinnen, S Vinckier, MS Soengas, P Carmeliet, A Noël, P Agostinis
Nature Communications, 2022-05-19;13(1):2760.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ADAMTS2 and ADAMTS14 substitute ADAMTS3 in adults for proVEGFC activation and lymphatic homeostasis
Authors: L Dupont, L Joannes, F Morfoisse, S Blacher, C Monseur, CF Deroanne, A Noël, AC Colige
JCI Insight, 2022-04-22;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Remodeling lymphatic vessels in intrinsically aged skin on SKH-1 mouse using low dose 5-aminolevulinic acid photodynamic therapy via VEGF-C/VEGFR3 pathway
Authors: Y Yang, S Shen, Y Cao, D Wang, Z Kang, P Wang, X Wang
Photodiagnosis and photodynamic therapy, 2022-04-06;0(0):102851.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiogenic and molecular diversity determine hepatic melanoma metastasis and response to anti-angiogenic treatment
Authors: SA Wohlfeil, V Häfele, B Dietsch, C Weller, C Sticht, AS Jauch, M Winkler, CD Schmid, AL Irkens, A Olsavszky, K Schledzews, PS Reiners-Ko, S Goerdt, C Géraud
Journal of Translational Medicine, 2022-02-02;20(1):62.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models
Authors: RJ Torphy, Y Sun, R Lin, A Caffrey-Ca, Y Fujiwara, F Ho, EN Miller, MD McCarter, TR Lyons, RD Schulick, RM Kedl, Y Zhu
Nature Communications, 2022-01-10;13(1):97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone marrow sinusoidal endothelium controls terminal erythroid differentiation and reticulocyte maturation
Authors: J Heil, V Olsavszky, K Busch, K Klapproth, C de la Torr, C Sticht, K Sandorski, J Hoffmann, H Schönhaber, J Zierow, M Winkler, CD Schmid, T Staniczek, DE Daniels, J Frayne, G Metzgeroth, D Nowak, S Schneider, M Neumaier, V Weyer, C Groden, HJ Gröne, K Richter, C Mogler, MM Taketo, K Schledzews, C Géraud, S Goerdt, PS Koch
Nature Communications, 2021-11-29;12(1):6963.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Npr2 mutant mice show vasodilation and undeveloped adipocytes in mesentery
Authors: C Sogawa-Fuj, Y Fujiwara, A Hanagata, Q Yang, T Mihara, N Kaji, T Kunieda, M Hori
Bmc Research Notes, 2021-11-27;14(1):438.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Expanded renal lymphatics improve recovery following kidney injury
Authors: G Baranwal, HA Creed, LM Black, A Auger, AM Quach, R Vegiraju, HE Eckenrode, A Agarwal, JM Rutkowski
Physiological Reports, 2021-11-01;9(22):e15094.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities
Authors: E Redder, N Kirschnick, S Bobe, R Hägerling, NR Hansmeier, F Kiefer
PLoS ONE, 2021-09-20;16(9):e0249256.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
KRAS-driven model of Gorham-Stout disease effectively treated with trametinib
Authors: N Homayun Se, AL McCarter, R Helaers, C Galant, LM Boon, P Brouillard, M Vikkula, MT Dellinger
JCI Insight, 2021-08-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
3-hydroxy-L-kynurenamine is an immunomodulatory biogenic amine
Authors: CC Clement, A D'Alessand, S Thangaswam, S Chalmers, R Furtado, S Spada, G Mondanelli, F Ianni, S Gehrke, M Gargaro, G Manni, LCL Cara, P Runge, WL Tsai, S Karaman, J Arasa, R Fernandez-, A Beck, A Macchiarul, M Gadina, C Halin, F Fallarino, M Skobe, M Veldhoen, S Moretti, S Formenti, S Demaria, RK Soni, R Galarini, R Sardella, G Lauvau, C Putterman, K Alitalo, U Grohmann, L Santambrog
Nature Communications, 2021-07-21;12(1):4447.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema
Authors: D Sz?ke, G Kovács, É Kemecsei, L Bálint, K Szoták-Ajt, P Aradi, A Styevkóné, BL Mui, YK Tam, TD Madden, K Karikó, RP Kataru, MJ Hope, D Weissman, BJ Mehrara, N Pardi, Z Jakus
Nature Communications, 2021-06-08;12(1):3460.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-Cell Transcriptional Heterogeneity of Lymphatic Endothelial Cells in Normal and Inflamed Murine Lymph Nodes
Authors: E Sibler, Y He, L Ducoli, N Keller, N Fujimoto, LC Dieterich, M Detmar
Cells, 2021-06-02;10(6):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice
Authors: J Zhu, T Inomata, K Fujimoto, K Uchida, K Fujio, K Nagino, M Miura, N Negishi, Y Okumura, Y Akasaki, K Hirosawa, M Kuwahara, A Eguchi, H Shokirova, A Yanagawa, A Midorikawa, A Murakami
Investigative Ophthalmology & Visual Science, 2021-06-01;62(7):3.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
New lymphatic cell formation is associated with damaged brain tissue clearance after penetrating traumatic brain injury
Authors: FW Meng, JT Yu, JY Chen, PF Yang
Scientific Reports, 2021-05-13;11(1):10193.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mesenchymal stem cells induce tumor stroma formation and epithelial?mesenchymal transition through SPARC expression in colorectal cancer
Authors: T Naito, R Yuge, Y Kitadai, H Takigawa, Y Higashi, T Kuwai, K Kuraoka, S Tanaka, K Chayama
Oncology reports, 2021-04-28;45(6):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation
Authors: H Shokirova, T Inomata, T Saitoh, J Zhu, K Fujio, Y Okumura, A Yanagawa, K Fujimoto, J Sung, A Eguchi, M Miura, K Nagino, K Hirosawa, M Kuwahara, Y Akasaki, H Nagase, A Murakami
Scientific Reports, 2021-04-21;11(1):8647.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A
Authors: R Luo, Y Cheng, D Chang, T Liu, L Liu, G Pei, N Zhang, Z Wang, K Guo, W Chen, M Li, L Fan, C Zhang, Y Li, W Dai, M Zuo, Y Xu, Y Yao, S Ge, G Xu
Theranostics, 2021-01-01;11(1):117-131.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Blood and lymphatic systems are segregated by the FLCN tumor suppressor
Authors: I Tai-Nagara, Y Hasumi, D Kusumoto, H Hasumi, K Okabe, T Ando, F Matsuzaki, F Itoh, H Saya, C Liu, W Li, YS Mukouyama, W Marston Li, X Liu, M Hirashima, Y Suzuki, S Funasaki, Y Satou, M Furuya, M Baba, Y Kubota
Nature Communications, 2020-12-09;11(1):6314.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Novel immunotherapeutic effects of topically administered ripasudil (K-115) on corneal allograft survival
Authors: T Inomata, K Fujimoto, Y Okumura, J Zhu, K Fujio, H Shokirova, M Miura, M Okano, T Funaki, J Sung, N Negishi, A Murakami
Sci Rep, 2020-11-13;10(1):19817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Eye lymphatic defects induced by bone morphogenetic protein 9 deficiency have no functional consequences on intraocular pressure
Authors: M Subileau, N Acar, A Carret, L Bretillon, I Vilgrain, S Bailly, D Vittet
Sci Rep, 2020-09-29;10(1):16040.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth
Authors: Krivanek J, Soldatov RA, Kastriti ME et al.
Nature Communications
-
Defective development and microcirculation of intestine in Npr2 mutant mice
Authors: C Sogawa-Fuj, A Hanagata, Y Fujiwara, Y Ishida, H Tomiyasu, T Kunieda, H Nakatomi, M Hori
Sci Rep, 2020-09-08;10(1):14761.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of receptor activity-modifying protein 1 suppresses the development of endometriosis and the formation of blood and lymphatic vessels
Authors: M Honda, Y Ito, K Hattori, K Hosono, K Sekiguchi, K Tsujikawa, N Unno, M Majima
J. Cell. Mol. Med., 2020-09-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling
Authors: X Geng, K Yanagida, RG Akwii, D Choi, L Chen, Y Ho, B Cha, MR Mahamud, K Berman de, H Ichise, H Chen, J Wythe, CM Mikelis, T Hla, RS Srinivasan
JCI Insight, 2020-07-23;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characterizing Lymphangiogenesis and Concurrent Inflammation in Adipose Tissue in Response to VEGF-D
Authors: A Chakrabort, CK Scogin, K Rizwan, TS Morley, JM Rutkowski
Front Physiol, 2020-04-22;11(0):363.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Progesterone Receptor Serves the Ovary as a Trigger of Ovulation and a Terminator of Inflammation
Authors: CJ Park, PC Lin, S Zhou, R Barakat, ST Bashir, JM Choi, JA Cacioppo, OR Oakley, DM Duffy, JP Lydon, CJ Ko
Cell Rep, 2020-04-14;31(2):107496.
Species: Mouse
Sample Types: Granulosa Cells
Applications: IHC -
A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics
Authors: Lioux G, Liu X, Temi�o S et al.
Developmental Cell
-
VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours
Authors: E Song, T Mao, H Dong, LSB Boisserand, S Antila, M Bosenberg, K Alitalo, JL Thomas, A Iwasaki
Nature, 2020-01-15;577(7792):689-694.
Species: Mouse
Sample Types: Tissue
Applications: IF -
Receptor?interacting serine/threonine?protein kinase 1 promotes the progress and lymph metastasis of gallbladder cancer
Authors: G Zhu, Q Du, X Chen, X Wang, N Tang, F She, Y Chen
Oncol. Rep., 2019-09-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth
Authors: SY Yoon, LC Dieterich, S Karaman, ST Proulx, SB Bachmann, C Sciaroni, M Detmar
PLoS ONE, 2019-07-25;14(7):e0220341.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Placental chemokine compartmentalisation: A novel mammalian molecular control mechanism
Authors: KM Lee, GJ Wilson, M Pingen, A Fukuoka, CAH Hansell, R Bartolini, L Medina-Rui, GJ Graham
PLoS Biol., 2019-05-29;17(5):e3000287.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
Compartmentalized gut lymph node drainage dictates adaptive immune responses
Authors: D Esterházy, MCC Canesso, L Mesin, PA Muller, TBR de Castro, A Lockhart, M ElJalby, AMC Faria, D Mucida
Nature, 2019-04-15;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage
Authors: HO Reed, L Wang, J Sonett, M Chen, J Yang, L Li, P Aradi, Z Jakus, J D'Armiento, WW Hancock, ML Kahn
J. Clin. Invest., 2019-04-04;129(6):2514-2526.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Associating liver partition and portal vein ligation for staged hepatectomy: establishment of an animal model with insufficient liver remnant
Authors: A Dili, V Lebrun, C Bertrand, IA Leclercq
Lab. Invest., 2019-01-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Bone Morphogenetic Protein 9 Regulates Early Lymphatic-Specified Endothelial Cell Expansion during Mouse Embryonic Stem Cell Differentiation
Authors: M Subileau, G Merdzhanov, D Ciais, V Collin-Fau, JJ Feige, S Bailly, D Vittet
Stem Cell Reports, 2018-12-27;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy
Authors: E Gucciardo, S Loukovaara, P Salven, K Lehti
Int J Mol Sci, 2018-12-13;19(12):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
uPARAP/Endo180 receptor is a gatekeeper of VEGFR-2/VEGFR-3 heterodimerisation during pathological lymphangiogenesis
Authors: T Durré, F Morfoisse, C Erpicum, M Ebroin, S Blacher, M García-Cab, C Deroanne, T Louis, C Balsat, M Van de Vel, S Kaijalaine, F Kridelka, L Engelholm, I Struman, K Alitalo, N Behrendt, J Paupert, A Noel
Nat Commun, 2018-12-05;9(1):5178.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CD4+ T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema
Authors: GD García Nor, CL Ly, DA Cuzzone, RP Kataru, GE Hespe, JS Torrisi, JJ Huang, JC Gardenier, IL Savetsky, MD Nitti, JZ Yu, S Rehal, BJ Mehrara
Nat Commun, 2018-05-17;9(1):1970.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
RIP1 regulates TNF-?-mediated lymphangiogenesis and lymphatic metastasis in gallbladder cancer by modulating the NF-?B-VEGF-C pathway
Authors: CZ Li, XJ Jiang, B Lin, HJ Hong, SY Zhu, L Jiang, XQ Wang, NH Tang, FF She, YL Chen
Onco Targets Ther, 2018-05-16;11(0):2875-2890.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-C promotes the development of lymphatics in bone and bone loss
Authors: D Hominick, A Silva, N Khurana, Y Liu, PC Dechow, JQ Feng, B Pytowski, JM Rutkowski, K Alitalo, MT Dellinger
Elife, 2018-04-05;7(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Mir-126 is a conserved modulator of lymphatic development
Authors: Z Kontarakis, A Rossi, S Ramas, MT Dellinger, DYR Stainier
Dev. Biol., 2018-03-15;0(0):.
Species: Mouse
Sample Types: Embryo
Applications: Western Blot -
Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain 17D-204
Authors: LKM Lam, AM Watson, KD Ryman, WB Klimstra
Npj Vaccines, 2018-01-23;3(0):5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Indian Hedgehog Suppresses a Stromal Cell-Driven Intestinal Immune Response
Authors: BF Westendorp, NVJA Büller, ON Karpus, WA van Dop, J Koster, R Versteeg, PJ Koelink, CY Snel, S Meisner, JJTH Roelofs, A Uhmann, E Ver Loren, J Heijmans, H Hahn, V Muncan, ME Wildenberg, GR van den Br
Cell Mol Gastroenterol Hepatol, 2017-09-05;5(1):67-82.e1.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases
Authors: M García-Cab, J Paupert, S Blacher, M Van de Vel, AR Quesada, MA Medina, A Noël
J Hematol Oncol, 2017-06-19;10(1):122.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
cIAP2 promotes gallbladder cancer invasion and lymphangiogenesis by activating the NF-?B pathway
Authors: X Jiang, C Li, B Lin, H Hong, L Jiang, S Zhu, X Wang, N Tang, X Li, F She, Y Chen
Cancer Sci., 2017-05-31;108(6):1144-1156.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mechanotransduction activates canonical Wnt/?-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves
Authors: Boksik Cha
Genes Dev, 2016-06-16;30(12):1454-69.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD
J Clin Invest, 2016-05-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
CHD4-regulated plasmin activation impacts lymphovenous hemostasis and hepatic vascular integrity
J Clin Invest, 2016-05-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunoprecipitation -
Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation
Proc Natl Acad Sci USA, 2016-05-02;113(20):E2842-51.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Authors: Zhou Y, Williams J, Smallwood P, Nathans J
PLoS ONE, 2015-12-02;10(12):e0143650.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development.
Authors: Geng X, Cha B, Mahamud M, Lim K, Silasi-Mansat R, Uddin M, Miura N, Xia L, Simon A, Engel J, Chen H, Lupu F, Srinivasan R
Dev Biol, 2015-11-02;409(1):218-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymph flow regulates collecting lymphatic vessel maturation in vivo.
Authors: Sweet D, Jimenez J, Chang J, Hess P, Mericko-Ishizuka P, Fu J, Xia L, Davies P, Kahn M
J Clin Invest, 2015-07-27;125(8):2995-3007.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research.
Authors: Bianchi R, Teijeira A, Proulx S, Christiansen A, Seidel C, Rulicke T, Makinen T, Hagerling R, Halin C, Detmar M
PLoS ONE, 2015-04-07;10(4):e0122976.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Bone marrow-derived mesenchymal stem cells drive lymphangiogenesis.
Authors: Maertens L, Erpicum C, Detry B, Blacher S, Lenoir B, Carnet O, Pequeux C, Cataldo D, Lecomte J, Paupert J, Noel A
PLoS ONE, 2014-09-15;9(9):e106976.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease.
Authors: D'Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S
J Clin Invest, 2014-08-08;124(9):3863-78.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC -
Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process.
Authors: Kizhatil, Krishnak, Ryan, Margaret, Marchant, Jeffrey, Henrich, Stephen, John, Simon W
PLoS Biol, 2014-07-22;12(7):e1001912.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymphatic function is required prenatally for lung inflation at birth.
Authors: Jakus Z, Gleghorn J, Enis D, Sen A, Chia S, Liu X, Rawnsley D, Yang Y, Hess P, Zou Z, Yang J, Guttentag S, Nelson C, Kahn M
J Exp Med, 2014-04-14;211(5):815-26.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro.
Authors: Iwasaki T, Takeda Y, Maruyama K, Yokosaki Y, Tsujino K, Tetsumoto S, Kuhara H, Nakanishi K, Otani Y, Jin Y, Kohmo S, Hirata H, Takahashi R, Suzuki M, Inoue K, Nagatomo I, Goya S, Kijima T, Kumagai T, Tachibana I, Kawase I, Kumanogoh A
J Biol Chem, 2012-12-05;288(4):2118-31.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Selection -
Rho-family GTPase Cdc42 controls migration of Langerhans cells in vivo.
Authors: Luckashenak N, Wahe A, Breit K, Brakebusch C, Brocker T
J Immunol, 2012-12-03;190(1):27-35.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiopoietin-1 is essential in mouse vasculature during development and in response to injury.
Authors: Jeansson M, Gawlik A, Anderson G
J. Clin. Invest., 2011-05-23;121(6):2278-89.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Sphingosine 1-Phosphate-Induced Motility and Endocytosis of Dendritic Cells Is Regulated by SWAP-70 through RhoA.
Authors: Ocana-Morgner C, Reichardt P, Chopin M, Braungart S, Wahren C, Gunzer M, Jessberger R
J. Immunol., 2011-03-18;186(9):5345-55.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3.
Authors: Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A
J. Cell Biol., 2010-01-11;188(1):115-30.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
ALK1 signaling regulates early postnatal lymphatic vessel development.
Authors: Niessen K, Zhang G, Ridgway JB, Chen H, Yan M
Blood, 2009-11-10;115(8):1654-61.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth.
Authors: Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J
Proc. Natl. Acad. Sci. U.S.A., 2009-04-09;106(17):7137-42.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes.
Authors: Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T
EMBO J., 2009-04-02;28(9):1319-31.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs.
Authors: Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A
J. Immunol., 2008-11-01;181(9):6189-200.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice.
Authors: Zhang J, Dong H, Wang B, Zhu S, Croy BA
Biol. Reprod., 2008-05-07;79(3):450-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Thymic stromal lymphopoietin transgenic mice develop cryoglobulinemia and hepatitis with similarities to human hepatitis C liver disease.
Authors: Kowalewska J, Muhlfeld AS, Hudkins KL, Yeh MM, Farr AG, Ravetch JV, Alpers CE
Am. J. Pathol., 2007-03-01;170(3):981-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy.
Authors: Bagley RG, Weber W, Rouleau C, Teicher BA
Cancer Res., 2005-11-01;65(21):9741-50.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse LYVE-1 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Mouse LYVE-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
In paraffin embedded sample.
Antigen retrieval with Tris-EDTA Buffer pH 9.0 and fixation with Methanol.
Blocking with TBS 2% BSA -0.5% Triton X-100.
Incubation of Lyve1 (AF2125) at a dilution of 1:400 (O/N at 4C).
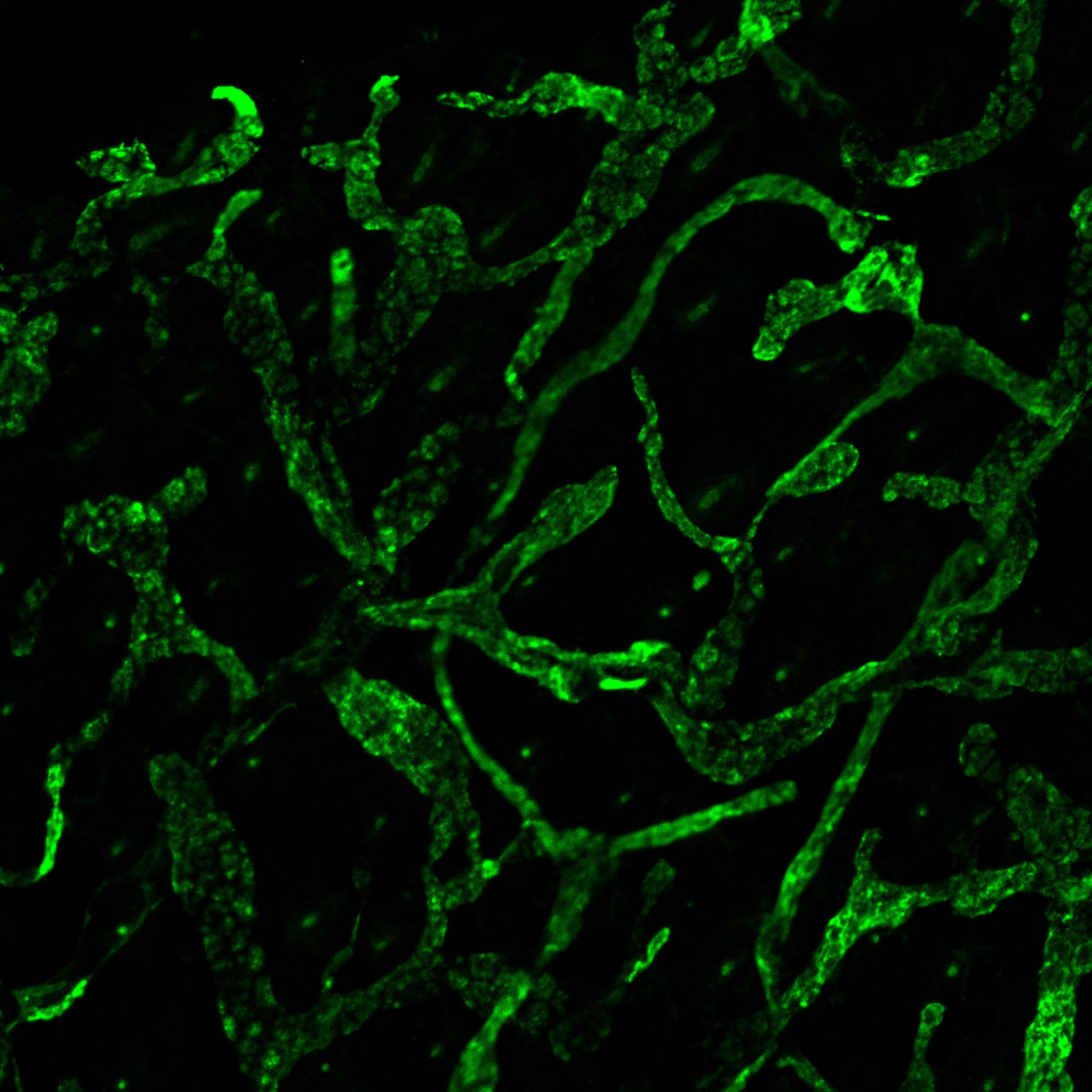
Mouse ear dermis was fixed in 2%PFA and blocked/permeabilized overnight in TBS/5% donkey serum/0.5% Tx100 before incubation in AF2125 at a dilution of 1:250 (O/N at 4C). Antibody was then detected using an alexafluor 488 labeled donkey anti goat secondary.