Mouse VEGF164 Antibody Summary
Ala27-Arg190
Accession # AAA40547
Applications
Mouse VEGF Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
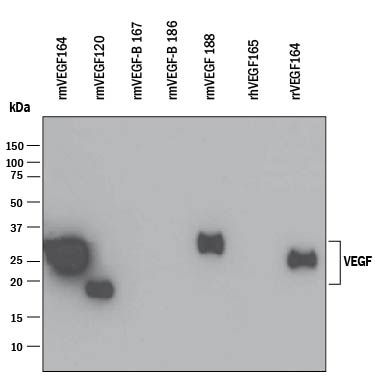 View Larger
View Larger
Detection of Recombinant Human, Mouse, and Rat VEGF by Western Blot. Western blot shows 25 ng of Recombinant Human VEGF165(Catalog # 293-VE), Recombinant Mouse VEGF164(Catalog # 493-MV), Recombinant Mouse VEGF120(Catalog # 494-VE), Recombinant Mouse VEGF-B167(Catalog # 2595-VE), Recombinant Mouse VEGF-B 186 (Catalog # 767-VE), Recombinant Mouse VEGF188(Catalog # 7916-MV), and Recombinant Rat VEGF164(Catalog # 564-RV). PVDF Membrane was probed with 0.1 µg/mL of Goat Anti-Mouse VEGF164Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-493-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). A specific band was detected for VEGF at approximately 18-27 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 3.
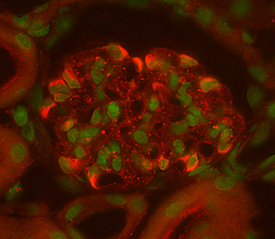 View Larger
View Larger
VEGF164in Mouse Kidney. Vascular Endothelial Growth Factor 164 (VEGF164) was detected in perfusion fixed frozen sections of mouse kidney using Goat Anti-Mouse VEGF164Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-493-NA) at 15 µg/mL overnight at 4 °C. Tissue was stained (red) and counterstained (green). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
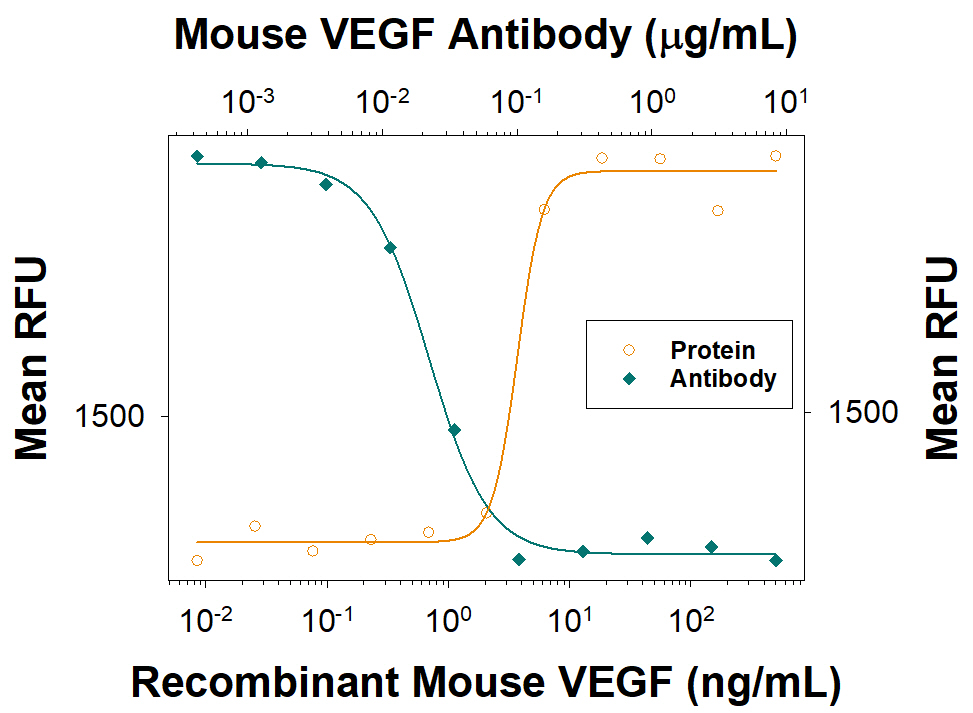 View Larger
View Larger
Cell Proliferation Induced by VEGF164 and Neutralization by Mouse VEGF Antibody. Recombinant Mouse VEGF164 (Catalog # 493-MV) stimulates proliferation in HUVEC human umbilical vein endothelial cells in a dose-dependent manner (orange line), as measured by Resazurin (AR002). Proliferation elicited by Recombinant Mouse VEGF164 (10 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Mouse VEGF164 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-493-NA). The ND50 is typically <0.15 µg/mL.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VEGF
Vascular Endothelial Growth Factor (VEGF or VEGF-A), also known as Vascular Permeability Factor (VPF), is a potent mediator of both angiogenesis and vasculogenesis in the fetus and adult. It is a member of the PDGF family that is characterized by the presence of eight conserved cysteine residues and a cystine knot structure. VEGF164 appears to be the most abundant and potent isoform, followed by VEGF120 and VEGF188. Mouse VEGF164 is an approximately 50 kDa molecular weight homodimer sharing 97% aa sequence identity with corresponding regions of rat, 89% with human and porcine, 90% with feline, equine and canine, and 88% with bovine VEGF, respectively. VEGF binds the type I transmembrane receptor tyrosine kinases VEGF R1 (also called Flt-1) and VEGF R2 (Flk-1/KDR) on endothelial cells. Although VEGF affinity is highest for binding to VEGF R1, VEGF R2 appears to be the primary mediator of VEGF angiogenic activity. Human VEGF165 binds the Semaphorin receptor, Neuropilin-1 and promotes complex formation with VEGF R2. VEGF is required during embryogenesis and functions to regulate the proliferation, migration, and survival of endothelial cells. In adults, VEGF functions mainly in wound healing and the female reproductive cycle. Pathologically, it is involved in tumor angiogenesis and vascular leakage. Circulating VEGF levels correlate with disease activity in autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus. VEGF is induced by hypoxia and cytokines such as IL-1, IL-6, IL-8, Oncostatin M (OSM) and TNF-alpha.
Product Datasheets
Citations for Mouse VEGF164 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
92
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The Ethyl Acetate Extract of Phyllanthus emblica L. Alleviates Diabetic Nephropathy in a Murine Model of Diabetes
Authors: Lin, CH;Shih, CC;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
A brainstem-hypothalamus neuronal circuit reduces feeding upon heat exposure
Authors: Benevento, M;Alpár, A;Gundacker, A;Afjehi, L;Balueva, K;Hevesi, Z;Hanics, J;Rehman, S;Pollak, DD;Lubec, G;Wulff, P;Prevot, V;Horvath, TL;Harkany, T;
Nature
Species: Murine polyomavirus strain A3, Viral
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Vertical Sleeve Gastrectomy Offers Protection against Disturbed Flow-Induced Atherosclerosis in High-Fat Diet-Fed Mice
Authors: JH Wei, WJ Lee, JL Luo, HL Huang, SC Wang, RH Chou, PH Huang, SJ Lin
International Journal of Molecular Sciences, 2023-03-16;24(6):.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone
Authors: E Lee, EA Lee, E Kong, H Chon, M Llaiqui-Co, CH Park, BY Park, NR Kang, JS Yoo, HS Lee, HS Kim, SH Park, SW Choi, D Vestweber, JH Lee, P Kim, WS Lee, I Kim
Experimental & Molecular Medicine, 2023-02-24;55(2):470-484.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Kallistatin prevents ovarian hyperstimulation syndrome by regulating vascular leakage
Authors: J Huang, Y Mao, Q Li, H Hong, N Tang, X Kang, Y Huang, J Liu, Q Gong, Y Yao, L Li
Journal of Cellular and Molecular Medicine, 2022-07-21;26(16):4613-4623.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Regenerating glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy
Authors: J Fu, T Shinjo, Q Li, R St-Louis, K Park, MG Yu, H Yokomizo, F Simao, Q Huang, IH Wu, GL King
JCI Insight, 2022-03-08;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Vascular endothelial growth factor-A/vascular endothelial growth factor2 signaling in spinal neurons contributes to bone cancer pain
Authors: LJ Fan, HM Kan, XT Chen, YY Sun, LP Chen, W Shen
Molecular pain, 2022-01-01;18(0):1744806922107.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Efficient protein incorporation and release by a jigsaw-shaped self-assembling peptide hydrogel for injured brain regeneration
Authors: A Yaguchi, M Oshikawa, G Watanabe, H Hiramatsu, N Uchida, C Hara, N Kaneko, K Sawamoto, T Muraoka, I Ajioka
Nature Communications, 2021-11-19;12(1):6623.
Species: Mouse
Sample Types: Protein
Applications: ELISA Capture -
VEGF Contributes to Mesenchymal Stem Cell-Mediated Reversion of Nor1-Dependent Hypertrophy in iPS Cell-Derived Cardiomyocytes
Authors: D Philipp, M Holthaus, V Basoah, K Pfannkuche, L Suhr, T Wahlers, A Paunel-Gör
Stem Cells International, 2021-04-10;2021(0):8888575.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: Neutralization, Western Blot -
PDGF Receptor Alpha Signaling Is Key for M�ller Cell Homeostasis Functions
Authors: N Díaz-Lezam, A Wolf, S Koch, AM Pfaller, J Biber, X Guillonnea, T Langmann, A Grosche
International Journal of Molecular Sciences, 2021-01-25;22(3):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Cancer immunotherapy via targeted TGF-&beta signalling blockade in TH cells
Authors: S Li, M Liu, MH Do, C Chou, EG Stamatiade, BG Nixon, W Shi, X Zhang, P Li, S Gao, KJ Capistrano, H Xu, NV Cheung, MO Li
Nature, 2020-10-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteocyte Vegf-a contributes to myeloma-associated angiogenesis and is regulated by Fgf23
Authors: PL Mulcrone, SKE Edwards, DN Petrusca, LS Haneline, J Delgado-Ca, GD Roodman
Sci Rep, 2020-10-14;10(1):17319.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Overexpression of microRNA-367 inhibits angiogenesis in ovarian cancer by downregulating the expression of LPA1
Authors: Q Zheng, X Dai, W Fang, Y Zheng, J Zhang, Y Liu, D Gu
Cancer Cell Int, 2020-10-02;20(0):476.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Activated FGF2 signaling pathway in tumor vasculature is essential for acquired resistance to anti-VEGF therapy
Authors: K Ichikawa, S Watanabe M, Y Minoshima, J Matsui, Y Funahashi
Sci Rep, 2020-02-19;10(1):2939.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Chitinase-3-Like-1 Promotes M2 Macrophage Differentiation and Induces Choroidal Neovascularization in Neovascular Age-Related Macular Degeneration
Authors: N Xu, Q Bo, R Shao, J Liang, Y Zhai, S Yang, F Wang, X Sun
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4596-4605.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Sustained intraocular vascular endothelial growth factor neutralisation does not affect retinal and choroidal vasculature in Ins2Akita diabetic mice
Authors: Judith Lechner, Jose R Hombrebueno, Edoardo Pedrini, Mei Chen, Heping Xu
Diabetes and Vascular Disease Research
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Serum miRNAs are potential biomarkers for the detection of disc degeneration, among which miR-26a-5p suppresses Smad1 to regulate disc homeostasis
Authors: Y Fan, L Zhao, W Xie, D Yi, S He, D Chen, J Huang
J. Cell. Mol. Med., 2019-07-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Protease-activated receptor 2 protects against VEGF inhibitor-induced glomerular endothelial and podocyte injury
Authors: Y Oe, T Fushima, E Sato, A Sekimoto, K Kisu, H Sato, J Sugawara, S Ito, N Takahashi
Sci Rep, 2019-02-27;9(1):2986.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Osteoblasts are "educated" by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment
Authors: AD Kolb, AB Shupp, D Mukhopadhy, FC Marini, KM Bussard
Breast Cancer Res., 2019-02-27;21(1):31.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC
Authors: B John, C Naczki, C Patel, A Ghoneum, S Qasem, Z Salih, N Said
Oncogene, 2019-02-14;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Co-inhibition of PGF and VEGF blocks their expression in mononuclear phagocytes and limits neovascularization and leakage in the murine retina
Authors: C Balser, A Wolf, M Herb, T Langmann
J Neuroinflammation, 2019-02-07;16(1):26.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Regeneration-associated cell transplantation contributes to tissue recovery in mice with acute ischemic stroke
Authors: T Nakayama, E Nagata, H Masuda, T Asahara, S Takizawa
PLoS ONE, 2019-01-25;14(1):e0210198.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone Morphogenetic Protein 9 Regulates Early Lymphatic-Specified Endothelial Cell Expansion during Mouse Embryonic Stem Cell Differentiation
Authors: M Subileau, G Merdzhanov, D Ciais, V Collin-Fau, JJ Feige, S Bailly, D Vittet
Stem Cell Reports, 2018-12-27;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
The AB loop of oncostatin M (OSM) determines species-specific signaling in humans and mice
Authors: JM Adrian-Seg, K Sreenivasa, P Gajawada, H Lörchner, T Braun, J Pöling
J. Biol. Chem., 2018-10-29;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Hapten-mediated recruitment of polyclonal antibodies to tumors engenders antitumor immunity
Authors: B Schrand, E Clark, A Levay, AR Capote, O Martinez, R Brenneman, I Castro, E Gilboa
Nat Commun, 2018-08-22;9(1):3348.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bushen Huoxue Recipe Alleviates Implantation Loss in Mice by Enhancing Estrogen-Progesterone Signals and Promoting Decidual Angiogenesis Through FGF2 During Early Pregnancy
Authors: J Ding, X Tan, K Song, W Ma, J Xiao, Y Song, M Zhang
Front Pharmacol, 2018-05-15;9(0):437.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis
Authors: T Kobori, S Hamasaki, A Kitaura, Y Yamazaki, T Nishinaka, A Niwa, S Nakao, H Wake, S Mori, T Yoshino, M Nishibori, H Takahashi
Front Immunol, 2018-03-06;9(0):334.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Vascularization via activation of VEGF-VEGFR signaling is essential for peripheral nerve regeneration
Authors: Y Nishida, Y Yamada, H Kanemaru, A Ohazama, T Maeda, K Seo
Biomed. Res., 2018-01-01;39(6):287-294.
Species: Mouse
Sample Types:
Applications: Neutralization -
Modulation of Salmonella Tumor-Colonization and Intratumoral Anti-angiogenesis by Triptolide and Its Mechanism
Authors: J Chen, Y Qiao, B Tang, G Chen, X Liu, B Yang, J Wei, X Zhang, X Cheng, P Du, W Jiang, Q Hu, ZC Hua
Theranostics, 2017-06-01;7(8):2250-2260.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Ferrochelatase is a therapeutic target for ocular neovascularization
Authors: HD Basavaraja, RS Sulaiman, X Qi, T Shetty, S Sheik Pran, KL Sishtla, B Lee, J Quigley, S Alkhairy, CM Briggs, K Gupta, B Tang, M Shadmand, MB Grant, ME Boulton, SY Seo, TW Corson
EMBO Mol Med, 2017-06-01;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Functional Assay -
Control of metastatic niche formation by targeting APBA3/Mint3 in inflammatory monocytes
Authors: T Hara, HJ Nakaoka, T Hayashi, K Mimura, D Hoshino, M Inoue, F Nagamura, Y Murakami, M Seiki, T Sakamoto
Proc. Natl. Acad. Sci. U.S.A., 2017-05-15;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis
Authors: XW Chen, TJ Yu, J Zhang, Y Li, HL Chen, GF Yang, W Yu, YZ Liu, XX Liu, CF Duan, HL Tang, M Qiu, CL Wang, H Zheng, J Yue, AM Guo, J Yang
Oncogene, 2017-05-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Functional Assay -
VEGF as a Paracrine Regulator of Conventional Outflow Facility
Authors: E Reina-Torr, JC Wen, KC Liu, G Li, JM Sherwood, JY Chang, P Challa, CM Flügel-Koc, WD Stamer, RR Allingham, DR Overby
Invest. Ophthalmol. Vis. Sci., 2017-03-01;58(3):1899-1908.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A delay in vascularization induces abnormal astrocyte proliferation and migration in the mouse retina
Authors: Akane Morita
Dev. Dyn, 2017-02-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
The biomechanical properties of an epithelial tissue determine the location of its vasculature
Nat Commun, 2016-12-20;7(0):13560.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PDGF-CC underlies resistance to VEGF-A inhibition and combinatorial targeting of both suppresses pathological angiogenesis more efficiently
Authors: L Zheng, C Zhao, Y Du, X Lin, Y Jiang, C Lee, G Tian, J Mi, X Li, Q Chen, Z Ye, L Huang, S Wang, X Ren, L Xing, W Chen, D Huang, Z Gao, S Zhang, W Lu, Z Tang, B Wang, R Ju, X Li
Oncotarget, 2016-11-22;7(47):77902-77915.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration
Authors: Ji-Hua Chen
Biomaterials, 2016-10-31;113(0):203-216.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Retinal pigment epithelial cell expression of active Rap 1a by scAAV2 inhibits choroidal neovascularization
Mol Ther Methods Clin Dev, 2016-08-24;3(0):16056.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Morphine decreases the pro-angiogenic interaction between breast cancer cells and macrophages in vitro
Sci Rep, 2016-08-12;6(0):31572.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Antihypoxic Potentiation of Standard Therapy for Experimental Colorectal Liver Metastasis through Myo-Inositol Trispyrophosphate
Clin Cancer Res, 2016-08-03;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy
Sci Rep, 2016-05-05;6(0):25509.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Endothelial Cords Promote Tumor Initial Growth prior to Vascular Function through a Paracrine Mechanism
Authors: C Zhao, W Zhang, Y Zhao, Y Yang, H Luo, G Ji, E Dong, H Deng, S Lin, Y Wei, H Yang
Sci Rep, 2016-01-14;6(0):19404.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1alpha activation.
Authors: Park S, Lee S, Kim H, Lee W, Hong K, Kim C
Eur J Immunol, 2015-01-15;45(4):1216-27.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Retinal neurons curb inflammation and enhance revascularization in ischemic retinopathies via proteinase-activated receptor-2.
Authors: Sitaras N, Rivera J, Noueihed B, Bien-Aime M, Zaniolo K, Omri S, Hamel D, Zhu T, Hardy P, Sapieha P, Joyal J, Chemtob S
Am J Pathol, 2014-12-03;185(2):581-95.
Species: Mouse
Sample Types: Whole Tissue
Applications: Neutralization -
Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis.
Authors: Bonapace L, Coissieux M, Wyckoff J, Mertz K, Varga Z, Junt T, Bentires-Alj M
Nature, 2014-10-22;515(7525):130-3.
Species: Mouse
Sample Types: In Vivo
-
Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis.
Authors: Lee E, Fertig E, Jin K, Sukumar S, Pandey N, Popel A
Nat Commun, 2014-09-02;5(0):4715.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC, Neutralization -
Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells.
Authors: Shelton M, Metz J, Liu J, Carpenedo R, Demers S, Stanford W, Skerjanc I
Stem Cell Reports, 2014-08-07;3(3):516-29.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: IHC -
RhoA/phosphatidylinositol 3-kinase/protein kinase B/mitogen-activated protein kinase signaling after growth arrest-specific protein 6/mer receptor tyrosine kinase engagement promotes epithelial cell growth and wound repair via upregulation of hepatocyte growth factor in macrophages.
Authors: Lee Y, Park H, Woo S, Park E, Kang J
J Pharmacol Exp Ther, 2014-06-17;350(3):563-77.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
An inducible hepatocellular carcinoma model for preclinical evaluation of antiangiogenic therapy in adult mice.
Authors: Runge A, Hu J, Wieland M, Bergeest J, Mogler C, Neumann A, Geraud C, Arnold B, Rohr K, Komljenovic D, Schirmacher P, Goerdt S, Augustin H
Cancer Res, 2014-06-06;74(15):4157-69.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Granzyme B releases vascular endothelial growth factor from extracellular matrix and induces vascular permeability.
Authors: Hendel, Alon, Hsu, Ivy, Granville, David J
Lab Invest, 2014-05-05;94(7):716-25.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC, IHC-P, Neutralization -
Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage.
Authors: Lee, Junyeop, Park, Dae-Youn, Park, Do Young, Park, Intae, Chang, Woohyok, Nakaoka, Yoshikaz, Komuro, Issei, Yoo, Ook-Joon, Koh, Gou Youn
Invest Ophthalmol Vis Sci, 2014-04-07;55(4):2191-9.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) in the brain.
Authors: Yu S, Levi L, Casadesus G, Kunos G, Noy N
J Biol Chem, 2014-03-18;289(18):12748-58.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Shiga toxin promotes podocyte injury in experimental hemolytic uremic syndrome via activation of the alternative pathway of complement.
Authors: Locatelli M, Buelli S, Pezzotta A, Corna D, Perico L, Tomasoni S, Rottoli D, Rizzo P, Conti D, Thurman J, Remuzzi G, Zoja C, Morigi M
J Am Soc Nephrol, 2014-02-27;25(8):1786-98.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dendritic cells: In vitro culture in two- and three-dimensional collagen systems and expression of collagen receptors in tumors and atherosclerotic microenvironments.
Authors: Sprague L, Muccioli M, Pate M, Singh M, Xiong C, Ostermann A, Niese B, Li Y, Li Y, Courreges M, Benencia F
Exp Cell Res, 2014-02-22;323(1):7-27.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Detection) -
IKK2 inhibition attenuates laser-induced choroidal neovascularization.
Authors: Lu H, Lu Q, Gaddipati S, Kasetti R, Wang W, Pasparakis M, Kaplan H, Li Q
PLoS ONE, 2014-01-28;9(1):e87530.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling.
Authors: Pushpakumar, Sathnur, Kundu, Sourav, Metreveli, Naira, Sen, Utpal
PLoS ONE, 2013-12-26;8(12):e83813.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor beta2 (TGF-beta2) pathways.
Authors: Di Bernardini E, Campagnolo P, Margariti A, Zampetaki A, Karamariti E, Hu Y, Xu Q
J Biol Chem, 2013-12-19;289(6):3383-93.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain.
Authors: Zeng, L, He, X, Wang, Y, Tang, Y, Zheng, C, Cai, H, Liu, J, Wang, Y, Fu, Y, Yang, G-Y
Gene Ther, 2013-10-24;21(1):37-43.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D.
Authors: Tan K, Chong S, Wong F, Evrard M, Tan S, Keeble J, Kemeny D, Ng L, Abastado J, Angeli V
Blood, 2013-10-10;122(22):3666-77.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
IL-18 regulates melanoma VLA-4 integrin activation through a Hierarchized sequence of inflammatory factors.
Authors: Valcarcel M, Carrascal T, Crende O, Vidal-Vanaclocha F
J Invest Dermatol, 2013-08-12;134(2):470-80.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
G protein-coupled receptor kinase 6 deficiency promotes angiogenesis, tumor progression, and metastasis.
Authors: Raghuwanshi S, Smith N, Rivers E, Thomas A, Sutton N, Hu Y, Mukhopadhyay S, Chen X, Leung T, Richardson R
J Immunol, 2013-04-15;190(10):5329-36.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development (Capture) -
The role of IL-1beta in the early tumor cell-induced angiogenic response.
Authors: Carmi Y, Dotan S, Rider P, Kaplanov I, White M, Baron R, Abutbul S, Huszar M, Dinarello C, Apte R, Voronov E
J Immunol, 2013-03-08;190(7):3500-9.
Species: Mouse
Sample Types: In Vivo, Matrigel Plug
Applications: IHC-Fr, Neutralization -
Inhibition of PAI-1 induces neutrophil-driven neoangiogenesis and promotes tissue regeneration via production of angiocrine factors in mice.
Blood, 2012-05-09;119(26):6382-93.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Response patterns of cytokines/chemokines in two murine strains after irradiation.
Authors: Zhang M, Yin L, Zhang K, Sun W, Yang S, Zhang B, Salzman P, Wang W, Liu C, Vidyasagar S, Zhang L, Ju S, Okunieff P, Zhang L
Cytokine, 2012-01-25;58(2):169-77.
Species: Mouse
Sample Types: Plasma
Applications: Luminex Development -
Relationship between complement membrane attack complex, chemokine (C-C motif) ligand 2 (CCL2) and vascular endothelial growth factor in mouse model of laser-induced choroidal neovascularization.
Authors: Liu J, Jha P, Lyzogubov VV, Tytarenko RG, Bora NS, Bora PS
J. Biol. Chem., 2011-04-22;286(23):20991-1001.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
IL-17 and VEGF are necessary for efficient corneal nerve regeneration.
Authors: Li Z, Burns AR, Han L, Rumbaut RE, Smith CW
Am. J. Pathol., 2011-03-01;178(3):1106-16.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC-Fr, Neutralization -
Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway.
Authors: Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Kach A, Humar B, Graf R, Moritz W, Clavien PA
Proc. Natl. Acad. Sci. U.S.A., 2011-01-31;108(7):2945-50.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction.
Authors: Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, Kobayashi K, Tongers J, Roncalli J, Tsurumi Y, Hagiwara N, Losordo DW
Proc. Natl. Acad. Sci. U.S.A., 2010-06-01;107(24):11008-13.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Platelet-derived growth factor-B normalizes micromorphology and vessel function in vascular endothelial growth factor-A-induced squamous cell carcinomas.
Authors: Lederle W, Linde N, Heusel J, Bzyl J, Woenne EC, Zwick S, Skobe M, Kiessling F, Fusenig NE, Mueller MM
Am. J. Pathol., 2009-12-30;176(2):981-94.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis.
Authors: Wuest TR, Carr DJ
J. Exp. Med., 2009-12-21;207(1):101-15, S1-2.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC, Neutralization -
Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease.
Authors: Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hahnel B, Grone HJ, Koesters R, Kriz W
Am. J. Pathol., 2009-10-15;175(5):1883-95.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution.
Authors: Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY
Blood, 2009-04-03;113(22):5650-9.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1.
Authors: Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, Grover AC, Ylaya K, Hewitt SM, Marx SJ, Spiegel AM, Libutti SK
Cancer Res., 2009-02-10;69(5):1858-66.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Safety profile of topical VEGF neutralization at the cornea.
Authors: Bock F, Onderka J, Rummelt C, Dietrich T, Bachmann B, Kruse FE, Schlotzer-Schrehardt U, Cursiefen C
Invest. Ophthalmol. Vis. Sci., 2009-01-17;50(5):2095-102.
Species: Mouse
Sample Types: Whole Tissue
Applications: Blocking -
Modulation of angiogenesis by a tetrameric tripeptide that antagonizes vascular endothelial growth factor receptor 1.
Authors: Ponticelli S, Marasco D, Tarallo V, Albuquerque RJ, Mitola S, Takeda A, Stassen JM, Presta M, Ambati J, Ruvo M, De Falco S
J. Biol. Chem., 2008-10-15;283(49):34250-9.
Species: Mouse
Sample Types: Recombinant Protein
Applications: ELISA Development -
Fibroblast-type reticular stromal cells regulate the lymph node vasculature.
Authors: Chyou S, Ekland EH, Carpenter AC, Tzeng TC, Tian S, Michaud M, Madri JA, Lu TT
J. Immunol., 2008-09-15;181(6):3887-96.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Role of cell and matrix-bound VEGF isoforms in lens development.
Authors: Saint-Geniez M, Kurihara T, D'Amore PA
Invest. Ophthalmol. Vis. Sci., 2008-08-29;50(1):311-21.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Regulation of scar formation by vascular endothelial growth factor.
Authors: Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, DiPietro LA
Lab. Invest., 2008-04-21;88(6):579-90.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Loss of vascular endothelial growth factor expression reduces vascularization, but not growth, of tumors lacking the Von Hippel-Lindau tumor suppressor gene.
Authors: Blouw B, Haase VH, Song H, Bergers G, Johnson RS
Oncogene, 2007-02-05;26(31):4531-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells.
Authors: Lederle W, Stark HJ, Skobe M, Fusenig NE, Mueller MM
Am. J. Pathol., 2006-11-01;169(5):1767-83.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function.
Authors: Kajiya K, Hirakawa S, Detmar M
Am. J. Pathol., 2006-10-01;169(4):1496-503.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta.
Authors: Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI
Genes Dev., 2006-09-15;20(18):2527-38.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Mast cells facilitate local VEGF release as an early event in the pathogenesis of postoperative peritoneal adhesions.
Authors: Cahill RA, Wang JH, Soohkai S, Redmond HP
Surgery, 2006-07-01;140(1):108-12.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain.
Authors: Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL
Nat. Neurosci., 2006-02-05;9(3):340-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A.
Authors: Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J
J. Clin. Invest., 2006-02-01;116(2):422-9.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Application of plasmid DNA encoding IL-18 diminishes development of herpetic stromal keratitis by antiangiogenic effects.
Authors: Kim B, Lee S, Suvas S, Rouse BT
J. Immunol., 2005-07-01;175(1):509-16.
Species: Mouse
Sample Types: Cell Lysates
Applications: ELISA Development -
Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation.
Authors: Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP
J. Immunol., 2005-01-01;174(1):215-22.
Species: Mouse
Sample Types: Cell Lysates
Applications: Neutralization -
Vascular endothelial growth factor (VEGF) gene transfer enhances surgical revascularization of necrotic bone.
Authors: Katsube K, Bishop AT, Simari RD, Yla-Herttuala S, Friedrich PF
J. Orthop. Res., 2004-11-30;23(2):469-74.
Species: Rabbit
Sample Types: Whole Tissue
Applications: IHC -
CXCR2-/- mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization.
Authors: Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT
J. Immunol., 2004-01-15;172(2):1237-45.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Estrogen regulates the production of VEGF for osteoclast formation and activity in op/op mice.
Authors: Kodama I, Sanada M, Yoshiko Y, Tsuda M, Ohama K
J. Bone Miner. Res., 2003-12-16;19(2):200-6.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Lack of alpha2-antiplasmin promotes pulmonary heart failure via overrelease of VEGF after acute myocardial infarction.
Authors: Kozawa O, Yoshimi N, Akamatsu S, Hara A, Mori H, Uematsu T
Blood, 2002-10-01;100(7):2487-93.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function.
Authors: Gabrilovich DI, 2018, Ishida T, Nadaf S, Ohm JE, Carbone DP
1537, 1999-10-01;5(10):2963-70.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization
FAQs
-
What band size(s) are detected when using AF-493-NA for Western blot?
This antibody detects Recombinant Mouse VEGF164 at 42 kDa under non-reducing conditions and 21 kDa under reducing conditions. Band sizes with natural samples may vary slightly depending on any post-translational modifications.
Reviews for Mouse VEGF164 Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Mouse VEGF164 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:





