Rat IgG1 Isotype Control Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
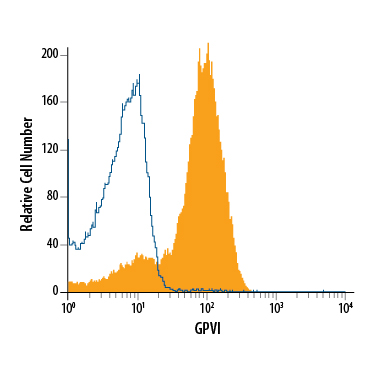
Detection of Rat IgG Control by Flow Cytometry Mouse platelets were stained with Rat Anti-Mouse GPVI Monoclonal Antibody (Catalog # MAB6758, filled histogram) or Rat IgG Isotype Control Antibody (Catalog # MAB005, open histogram), followed by Allophycocyanin-conjugated Anti-Rat IgG Secondary Antibody (Catalog F0113).
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: IgG1
R&D Systems offers a range of secondary antibodies and controls for flow cytometry, immunohistochemistry, and Western blotting. We provide species-specific secondary antibodies that are available with a variety of conjugated labels.
Our NorthernLights fluorescent secondary antibodies are bright and resistant to photobleaching. We are currently offering secondary antibodies recognizing mouse, rat, goat, sheep, and rabbit IgG as well as chicken IgY. These reagents are available with three distinct excitation and emission maxima, making them ideal for multi-color fluorescence microscopy.
Product Datasheets
Citations for Rat IgG1 Isotype Control
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
59
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses
Authors: T Hirz, S Mei, H Sarkar, Y Kfoury, S Wu, BM Verhoeven, AO Subtelny, DV Zlatev, MW Wszolek, K Salari, E Murray, F Chen, EZ Macosko, CL Wu, DT Scadden, DM Dahl, N Baryawno, PJ Saylor, PV Kharchenko, DB Sykes
Nature Communications, 2023-02-07;14(1):663.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo Control -
Leukotriene B4 Receptor 2 Mediates the Production of G-CSF That Plays a Critical Role in Steroid-Resistant Neutrophilic Airway Inflammation
Authors: DW Kwak, D Park, JH Kim
Biomedicines, 2022-11-19;10(11):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Single-cell transcriptomics reveals a senescence-associated IL-6/CCR6 axis driving radiodermatitis
Authors: M Paldor, O Levkovitch, D Eidelshtei, R Adar, CD Enk, Y Marmary, S Elgavish, Y Nevo, H Benyamini, I Plaschkes, S Klein, A Mali, S Rose-John, A Peled, E Galun, JH Axelrod
Oncogene, 2022-07-04;0(0):e15653.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo Control -
Semaphorin 3A regulates alveolar bone remodeling on orthodontic tooth movement
Authors: H Kamei, T Ishii, Y Nishii
Oncogene, 2022-06-02;12(1):9243.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization Control -
Chemokine CCL9 Is Upregulated Early in Chronic Kidney Disease and Counteracts Kidney Inflammation and Fibrosis
Authors: C Hemmers, C Schulte, J Wollenhaup, DWL Wong, E Harlacher, S Orth-Alamp, BM Klinkhamme, SH Schirmer, M Böhm, N Marx, T Speer, P Boor, J Jankowski, H Noels
Biomedicines, 2022-02-10;10(2):.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses
Authors: M Bruchard, M Geindreau, A Perrichet, C Truntzer, E Ballot, R Boidot, C Racoeur, E Barsac, F Chalmin, C Hibos, T Baranek, C Paget, B Ryffel, C Rébé, C Paul, F Végran, F Ghiringhel
Nature Immunology, 2022-01-31;23(2):262-274.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo Control -
Irradiated fibroblasts increase interleukin-6 expression and induce migration of head and neck squamous cell carcinoma
Authors: S Suzuki, S Toyoma, Y Kawasaki, T Yamada
PLoS ONE, 2022-01-28;17(1):e0262549.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization Control -
Granulocyte colony-stimulating factor (G-CSF) mediates bone resorption in periodontitis
Authors: H Yu, T Zhang, H Lu, Q Ma, D Zhao, J Sun, Z Wang
BMC Oral Health, 2021-06-12;21(1):299.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Liver-expressed Cd302 and Cr1l limit hepatitis C virus cross-species transmission to mice
Authors: RJP Brown, B Tegtmeyer, J Sheldon, T Khera, Anggakusum, D Todt, G Vieyres, R Weller, S Joecks, Y Zhang, S Sake, D Bankwitz, K Welsch, C Ginkel, M Engelmann, G Gerold, E Steinmann, Q Yuan, M Ott, FWR Vondran, T Krey, LJ Ströh, C Miskey, Z Ivics, V Herder, W Baumgärtne, C Lauber, M Seifert, AW Tarr, CP McClure, G Randall, Y Baktash, A Ploss, VLD Thi, E Michailidi, M Saeed, L Verhoye, P Meuleman, N Goedecke, D Wirth, CM Rice, T Pietschman
Sci Adv, 2020-11-04;6(45):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
p38&alpha in Macrophages Aggravates Arterial Endothelium Injury by Releasing IL-6 through phosphorylating Megakaryocytic Leukemia 1
Authors: M Zhang, J Gao, X Zhao, M Zhao, D Ma, X Zhang, D Tian, B Pan, X Yan, J Wu, X Meng, H Yin, L Zheng
Redox Biology, 2020-11-01;38(0):101775.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Toll-like Receptor 2 Facilitates Oxidative Damage-Induced Retinal Degeneration
Authors: K Mulfaul, E Ozaki, N Fernando, K Brennan, KR Chirco, E Connolly, C Greene, A Maminishki, RG Salomon, M Linetsky, R Natoli, RF Mullins, M Campbell, SL Doyle
Cell Rep, 2020-02-18;30(7):2209-2224.e5.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells
Authors: M Yu, A Malik Tyag, JY Li, J Adams, TL Denning, MN Weitzmann, RM Jones, R Pacifici
Nat Commun, 2020-01-24;11(1):468.
Species: Rat
Sample Types: In Vivo
Applications: In Vivo -
Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis
Authors: Y Takagaki, SM Lee, Z Dongqing, M Kitada, K Kanasaki, D Koya
Autophagy, 2020-01-22;0(0):1-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CCL28 promotes locomotor recovery after spinal cord injury via recruiting regulatory T cells
Authors: P Wang, X Qi, G Xu, J Liu, J Guo, X Li, X Ma, H Sun
Aging (Albany NY), 2019-09-26;11(18):7402-7415.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CCL20-CCR6 axis modulated traumatic brain injury-induced visual pathologies
Authors: M Das, X Tang, JY Han, K Mayilsamy, E Foran, MR Biswal, R Tzekov, SS Mohapatra, S Mohapatra
J Neuroinflammation, 2019-05-31;16(1):115.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Protein tyrosine phosphatase non-receptor type 22 modulates colitis in a microbiota-dependent manner
Authors: MR Spalinger, TS Schmidt, M Schwarzfis, L Hering, K Atrott, S Lang, C Gottier, A Geirnaert, C Lacroix, X Dai, DJ Rawlings, AC Chan, C von Mering, G Rogler, M Scharl
J. Clin. Invest., 2019-05-20;130(0):2527-2541.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC
Authors: B John, C Naczki, C Patel, A Ghoneum, S Qasem, Z Salih, N Said
Oncogene, 2019-02-14;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Modulating bone marrow hematopoietic lineage potential to prevent bone metastasis in breast cancer
Authors: JM Ubellacker, N Baryawno, N Severe, MJ DeCristo, J Sceneay, JN Hutchinson, MT Haider, CS Rhee, Y Qin, WM Gregory, AC Garrido-Ca, I Holen, JE Brown, RE Coleman, DT Scadden, SS McAllister
Cancer Res., 2018-07-31;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Estrogen Deficiency Promotes Cerebral Aneurysm Rupture by Upregulation of Th17 Cells and Interleukin-17A Which Downregulates E-Cadherin
Authors: BL Hoh, K Rojas, L Lin, HZ Fazal, S Hourani, KW Nowicki, MB Schneider, K Hosaka
J Am Heart Assoc, 2018-04-13;7(8):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models
Authors: Y Mita, K Nakayama, S Inari, Y Nishito, Y Yoshioka, N Sakai, K Sotani, T Nagamura, Y Kuzuhara, K Inagaki, M Iwasaki, H Misu, M Ikegawa, T Takamura, N Noguchi, Y Saito
Nat Commun, 2017-11-21;8(1):1658.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo Control -
Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis
Authors: JC Zhang, W Yao, C Dong, C Yang, Q Ren, M Ma, K Hashimoto
Transl Psychiatry, 2017-05-30;7(5):e1138.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Granulocyte colony-stimulating factor blockade enables dexamethasone to inhibit lipopolysaccharide-induced murine lung neutrophils
Authors: J Banuelos, Y Cao, SC Shin, BS Bochner, P Avila, S Li, X Jiang, MW Lingen, RP Schleimer, NZ Lu
PLoS ONE, 2017-05-19;12(5):e0177884.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Cooperation of Gastric Mononuclear Phagocytes with Helicobacter pylori during Colonization
Authors: M Viladomiu, J Bassaganya, N Tubau-Juni, B Kronsteine, A Leber, CW Philipson, V Zoccoli-Ro, R Hontecilla
J. Immunol, 2017-03-06;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway
Authors: SN Schock, NV Chandra, Y Sun, T Irie, Y Kitagawa, B Gotoh, L Coscoy, A Winoto
Cell Death Differ, 2017-01-06;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Autoimmune Renal Disease Is Exacerbated by S1P-Receptor-1-Dependent Intestinal Th17 Cell Migration to the Kidney
Authors: Ulf Panzer
Immunity, 2016-11-15;45(5):1078-1092.
Species: Mouse
Sample Types:
Applications: Neutralization -
Presentation of Cryptic Peptides by MHC Class I Is Enhanced by Inflammatory Stimuli
J Immunol, 2016-09-19;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer
Authors: MS Caetano, H Zhang, AM Cumpian, L Gong, N Unver, EJ Ostrin, S Daliri, SH Chang, CE Ochoa, S Hanash, C Behrens, II Wistuba, C Sternberg, H Kadara, CG Ferreira, SS Watowich, SJ Moghaddam
Cancer Res., 2016-04-01;76(11):3189-99.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury.
Authors: Bohannon J, Luan L, Hernandez A, Afzal A, Guo Y, Patil N, Fensterheim B, Sherwood E
J Leukoc Biol, 2015-11-04;99(4):629-40.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Mechanisms of Hypoxic Up-Regulation of Versican Gene Expression in Macrophages.
Authors: Sotoodehnejadnematalahi F, Staples K, Chrysanthou E, Pearson H, Ziegler-Heitbrock L, Burke B
PLoS ONE, 2015-06-09;10(6):e0125799.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
G-CSF Promotes Neuroblastoma Tumorigenicity and Metastasis via STAT3-Dependent Cancer Stem Cell Activation.
Authors: Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa L, Kim E, Shohet J
Cancer Res, 2015-04-23;75(12):2566-79.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency.
Authors: Abdel Fattah E, Bhattacharya A, Herron A, Safdar Z, Eissa N
J Immunol, 2015-04-17;194(11):5407-16.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
Coordinate expansion of murine hematopoietic and mesenchymal stem cell compartments by SHIPi.
Authors: Brooks R, Iyer S, Akada H, Neelam S, Russo C, Chisholm J, Kerr W
Stem Cells, 2015-03-01;33(3):848-58.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration.
Authors: Levy O, Calippe B, Lavalette S, Hu S, Raoul W, Dominguez E, Housset M, Paques M, Sahel J, Bemelmans A, Combadiere C, Guillonneau X, Sennlaub F
EMBO Mol Med, 2015-02-01;7(2):211-26.
Species: Mouse
Sample Types: In Vivo, Whole Cells, Whole Organism
Applications: In Vivo, Neutralization -
Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling.
Authors: Chandler, Ronald L, Damrauer, Jeffrey, Raab, Jesse R, Schisler, Jonathan, Wilkerson, Matthew, Didion, John P, Starmer, Joshua, Serber, Daniel, Yee, Della, Xiong, Jessie, Darr, David B, Pardo-Manuel de Villena, Fernando, Kim, William, Magnuson, Terry
Nat Commun, 2015-01-27;6(0):6118.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization Control -
Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation.
Authors: McKelvey R, Berta T, Old E, Ji R, Fitzgerald M
J Neurosci, 2015-01-14;35(2):457-66.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis.
Authors: Bonapace L, Coissieux M, Wyckoff J, Mertz K, Varga Z, Junt T, Bentires-Alj M
Nature, 2014-10-22;515(7525):130-3.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Syndecan-1 in the mouse parietal peritoneum microcirculation in inflammation.
Authors: Kowalewska P, Patrick A, Fox-Robichaud A
PLoS ONE, 2014-09-03;9(9):e104537.
Species: Mouse
Sample Types: In Vivo
Applications: IHC-Fr -
Targeting DNGR-1 (CLEC9A) with antibody/MUC1 peptide conjugates as a vaccine for carcinomas.
Authors: Picco G, Beatson R, Taylor-Papadimitriou J, Burchell J
Eur J Immunol, 2014-04-17;44(7):1947-55.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Leukocyte attraction by CCL20 and its receptor CCR6 in humans and mice with pneumococcal meningitis.
Authors: Klein M, Brouwer M, Angele B, Geldhoff M, Marquez G, Varona R, Hacker G, Schmetzer H, Hacker H, Hammerschmidt S, van der Ende A, Pfister H, van de Beek D, Koedel U
PLoS ONE, 2014-04-03;9(4):e93057.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The role of host and microbial factors in the pathogenesis of pneumococcal bacteraemia arising from a single bacterial cell bottleneck.
Authors: Gerlini A, Colomba L, Furi L, Braccini T, Manso A, Pammolli A, Wang B, Vivi A, Tassini M, Van Rooijen N, Pozzi G, Ricci S, Andrew P, Koedel U, Moxon E, Oggioni M
PLoS Pathog, 2014-03-20;10(3):e1004026.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity.
Authors: Ramkhelawon B, Hennessy E, Menager M, Ray T, Sheedy F, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner K, Moore K
Nat Med, 2014-03-02;20(4):377-84.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
G-CSF drives a posttraumatic immune program that protects the host from infection.
Authors: Gardner J, Noel J, Nikolaidis N, Karns R, Aronow B, Ogle C, McCormack F
J Immunol, 2014-01-27;192(5):2405-17.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Retinoic acid prevents mesenteric lymph node dendritic cells from inducing IL-13-producing inflammatory Th2 cells.
Authors: Yokota-Nakatsuma A, Takeuchi H, Ohoka Y, Kato C, Song S, Hoshino T, Yagita H, Ohteki T, Iwata M
Mucosal Immunol, 2013-11-13;7(4):786-801.
Species: Mouse
Sample Types: Whole Cells
Applications: Isotype Control -
Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-alpha antibodies.
Authors: Bhargava R, Altmann C, Andres-Hernando A, Webb R, Okamura K, Yang Y, Falk S, Schmidt E, Faubel S
PLoS ONE, 2013-11-12;8(11):e79037.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Microarray analyses demonstrate the involvement of type I interferons in psoriasiform pathology development in D6-deficient mice.
Authors: Baldwin H, Pallas K, King V, Jamieson T, McKimmie C, Nibbs R, Carballido J, Jaritz M, Rot A, Graham G
J Biol Chem, 2013-11-05;288(51):36473-83.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6.
Authors: Kim M, Gorouhi F, Ramirez S, Granick J, Byrne B, Soulika A, Simon S, Isseroff R
J Invest Dermatol, 2013-10-11;134(3):809-17.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure.
Authors: Kulkarni, Shilpa, Singh, Pankaj K, Ghosh, Sanchita, Posarac, Ana, Singh, Vijay K
Cytokine, 2013-04-03;62(2):278-85.
Species: Mouse
Sample Types: In Vivo
-
CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis.
Authors: Mabuchi T, Singh T, Takekoshi T, Jia G, Wu X, Kao M, Weiss I, Farber J, Hwang S
J Invest Dermatol, 2012-08-16;133(1):164-71.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
Canonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myeloma.
Authors: Blotta, Simona, Jakubikova, Jana, Calimeri, Teresa, Roccaro, Aldo M, Amodio, Nicola, Azab, Abdel Ka, Foresta, Umberto, Mitsiades, Constant, Rossi, Marco, Todoerti, Katia, Molica, Stefano, Morabito, Fortunat, Neri, Antonino, Tagliaferri, Piersand, Tassone, Pierfran, Anderson, Kenneth, Munshi, Nikhil C
Blood, 2012-07-20;120(25):5002-13.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry Control -
Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism.
Authors: Waight JD, Hu Q, Miller A, Liu S, Abrams SI
PLoS ONE, 2011-11-16;6(11):e27690.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice.
Authors: Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC
Nat. Med., 2011-04-24;17(5):581-8.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus.
Authors: Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, Kloke L, Heimann J, Gaber T, Brandenburg S, Scheffold A, Huehn J, Radbruch A, Burmester GR, Riemekasten G
Proc. Natl. Acad. Sci. U.S.A., 2009-12-14;107(1):204-9.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models.
Authors: Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N
Proc. Natl. Acad. Sci. U.S.A., 2009-04-03;106(16):6742-7.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
Rapid dendritic cell mobilization to the large intestinal epithelium is associated with resistance to Trichuris muris infection.
Authors: Cruickshank SM, Deschoolmeester ML, Svensson M, Howell G, Bazakou A, Logunova L, Little MC, English N, Mack M, Grencis RK, Else KJ, Carding SR
J. Immunol., 2009-03-01;182(5):3055-62.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
Tr1 regulatory T cells induced by ConA pretreatment prevent mice from ConA-induced hepatitis.
Authors: Ye F, Yan S, Xu L, Jiang Z, Liu N, Xiong S, Wang Y, Chu Y
Immunol. Lett., 2009-02-04;122(2):198-207.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice.
Authors: Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K
J. Immunol., 2007-12-15;179(12):8274-9.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
Anti-type II collagen antibody accelerates arthritis via CXCR2-expressing cells in IL-1 receptor antagonist-deficient mice.
Authors: Kagari T, Tanaka D, Doi H, Iwakura Y, Shimozato T
Eur. J. Immunol., 2007-10-01;37(10):2753-63.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
Junctional adhesion molecules (JAM)-B and -C contribute to leukocyte extravasation to the skin and mediate cutaneous inflammation.
Authors: Ludwig RJ, Zollner TM, Santoso S, Hardt K, Gille J, Baatz H, Johann PS, Pfeffer J, Radeke HH, Schon MP, Kaufmann R, Boehncke WH, Podda M
J. Invest. Dermatol., 2005-11-01;125(5):969-76.
Species: Mouse
Sample Types: In Vivo
Applications: Control -
MIP-1 gamma promotes receptor-activator-of-NF-kappa-B-ligand-induced osteoclast formation and survival.
Authors: Okamatsu Y, Battaglino R, Spate U, Stashenko P
J. Immunol., 2004-08-01;173(3):2084-90.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization, Neutralization Control
FAQs
-
What is the light chain of Rat IgG1 Isotype Control, Catalog # MAB005?
The light chain is kappa.
Reviews for Rat IgG1 Isotype Control
Average Rating: 4 (Based on 1 Review)
Have you used Rat IgG1 Isotype Control?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
