Recombinant Human FGF basic/FGF2/bFGF, 145 aa TC Grade, CF
Now offering a heat stable form of Recombinant Human FGF basic (Catalog # BT-FGFBHS) that retains activity at incubator temperatures and adds flexibility to your media change intervals.
Recombinant Human FGF basic/FGF2/bFGF, 145 aa TC Grade, CF Summary
Product Specifications
Ala144-Ser288
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
4114-TC
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl. |
| Reconstitution | Reconstitute at 0.5-1.0 mg/mL in sterile PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
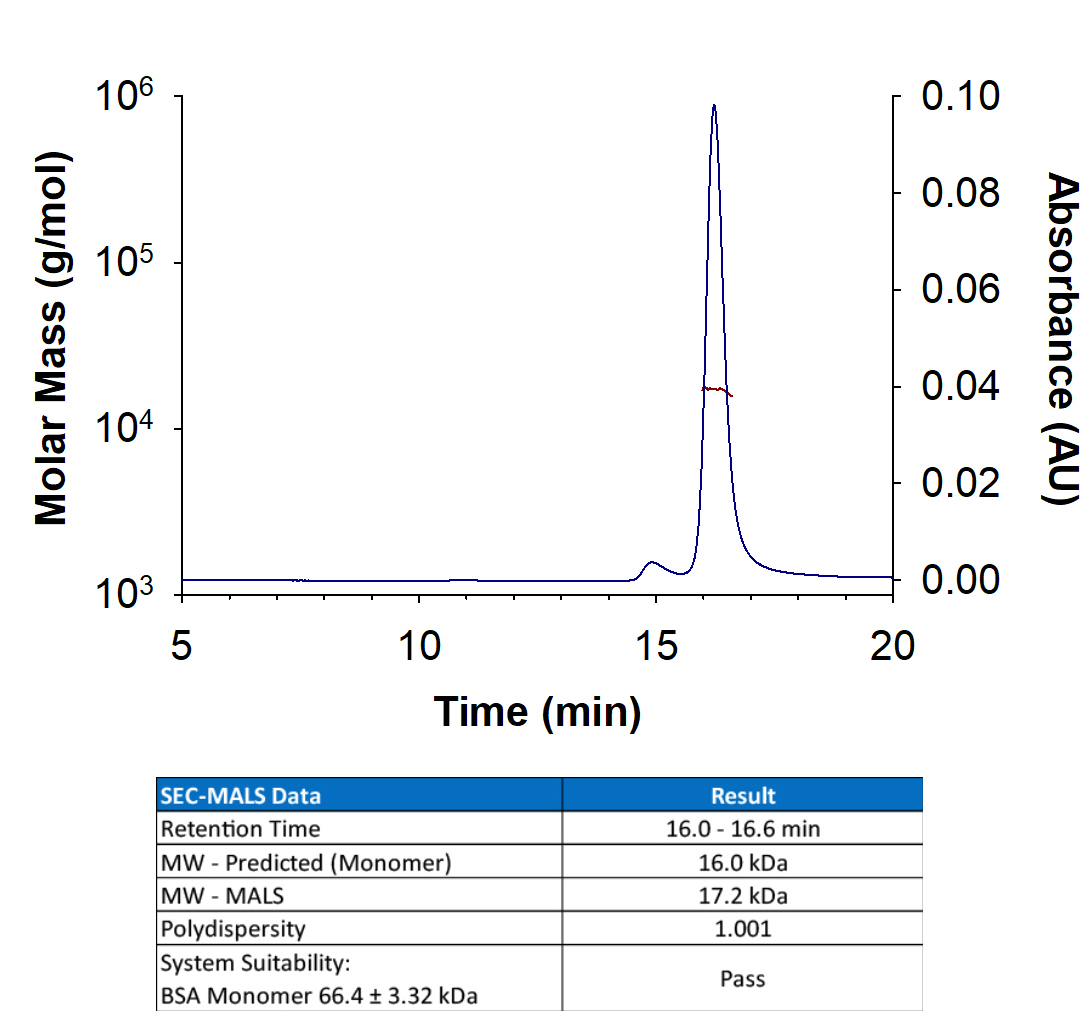 View Larger
View Larger
Recombinant human FGF basic/FGF2/bFGF, 145 aa TC Grade (Catalog # 4114-TC) has a molecular weight (MW) of 17.2 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
Reconstitution Calculator
Background: FGF basic/FGF2/bFGF
FGF basic (also known as FGF2 and HBGF-2) is an 18-34 kDa, heparin-binding member of the FGF superfamily of molecules (1-3). Superfamily members are characterized by the presence of a centrally placed beta -trefoil structure. FGF acidic (FGF1) and FGF basic (FGF2) were the first two identified FGFs, and the designations acidic and basic refer to their relative isoelectric points. Human FGF basic is 288 amino acids (aa) in length. There are multiple start sites, four of which utilize atypical CUG codons, and one that initiates at an AUG start site (4-6). The four CUG start sites generate high molecular weight (HMW) FGF basic. There is a 34 kDa, 288 aa form, a 24 kDa, 210 aa form, a 22.5 kDa, 201 aa form, and a 22 kDa, 196 aa form. All are retained intracellularly, undergo extensive methylation, and possess one or more nuclear localization signals (NLS) (7-9). The AUG initiating form is 18 kDa and 155 aa in length. There is no signal sequence (ss). It is, however, secreted directly through the plasma membrane via a mechanism that appears to be dependent upon tertiary structure (10). In place of a ss, there is purportedly a 9 aa N-terminal prosegment that precedes a 146 aa mature segment (11). Early isolations of 18 kDa bovine FGF basic yielded 146 aa molecules, an effect attributed to the presence of acid proteases (12). The molecule contains a heparin-binding site (aa residues 128-144), and undergoes phosphorylation at Ser117 (13). There is also an ill-defined C-terminal NLS that may be more “functional” (or 3-dimensional) than structural (7). Human 146 aa FGF basic is 97% aa identical to mouse FGF basic (14).
- Sorenson, V. et al. (2006) BioEssays 28:504.
- Kardami, E. et al. (2004) Cardiovasc. Res. 63:458.
- Nugent, M.A. and R.V. Lozzo (2000) Int. J. Biochem. Cell Biol. 32:115.
- Abraham, J.A. et al. (1986) EMBO J. 5:2523.
- Prats, H. et al. (1989) Proc. Natl. Acad. Sci. USA 86:1836.
- Arnaud, E. et al. (1999) Mol. Cell. Biol. 19:505.
- Foletti, A. et al. (2003) Cell. Mol. Life Sci. 60:2254.
- Arese, M. et al. (1999) Mol. Biol. Cell 10:1429.
- Pintucci, G. et al. (1996) Mol. Biol. Cell 7:1249.
- Nickel, W. (2005) Traffic 6:607.
- SwissProt # P09038.
- Klagsbrun, M. et al. (1987) Proc. Natl. Acad. Sci. USA 84:1839.
- Bailly, K. et al. (2000) FASEB J. 14:333.
- Hebert, J.M. et al. (1990) Dev. Biol. 138:454.
Citations for Recombinant Human FGF basic/FGF2/bFGF, 145 aa TC Grade, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
27
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
STAT3 activation of SCAP-SREBP-1 signaling upregulates fatty acid synthesis to promote tumor growth
Authors: Fan, Y;Zhang, R;Wang, C;Pan, M;Geng, F;Zhong, Y;Su, H;Kou, Y;Mo, X;Lefai, E;Han, X;Chakravarti, A;Guo, D;
The Journal of biological chemistry
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Cysteine Rich Intestinal Protein 2 is a copper-responsive regulator of skeletal muscle differentiation
Authors: Verdejo-Torres, O;Klein, DC;Novoa-Aponte, L;Carrazco-Carrillo, J;Bonilla-Pinto, D;Rivera, A;Fitisemanu, F;Jiménez-González, ML;Flinn, L;Pezacki, AT;Lanzirotti, A;Ortiz-Frade, LA;Chang, CJ;Navea, JG;Blaby-Haas, C;Hainer, SJ;Padilla-Benavides, T;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of Primary Cilium Length by O-GlcNAc during Neuronal Development in a Human Neuron Model
Authors: Tian, JL;Huang, CW;Eslami, F;Mannino, MP;Mai, RL;Hart, GW;
Cells
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Lysine catabolism reprograms tumour immunity through histone crotonylation
Authors: Yuan, H;Wu, X;Wu, Q;Chatoff, A;Megill, E;Gao, J;Huang, T;Duan, T;Yang, K;Jin, C;Yuan, F;Wang, S;Zhao, L;Zinn, PO;Abdullah, KG;Zhao, Y;Snyder, NW;Rich, JN;
Nature
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The oncogenic JAG1 intracellular domain is a transcriptional cofactor that acts in concert with DDX17/SMAD3/TGIF2
Authors: EJ Kim, JY Kim, SO Kim, N Hong, SH Choi, MG Park, J Jang, SW Ham, S Seo, SY Lee, K Lee, HJ Jeong, SJ Kim, S Jeong, K Min, SC Kim, X Jin, SH Kim, SH Kim, H Kim
Cell Reports, 2022-11-22;41(8):111626.
Species: Human, Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Alpha synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether-phospholipid membrane composition
Authors: L Mahoney-Sa, H Bouchaoui, I Boussaad, A Jonneaux, K Timmerman, O Berdeaux, S Ayton, R Krüger, JA Duce, D Devos, JC Devedjian
Cell Reports, 2022-08-23;40(8):111231.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Bioassay -
Sox9 directs divergent epigenomic states in brain tumor subtypes
Authors: D Sardar, HC Chen, A Reyes, S Varadharaj, A Jain, C Mohila, R Curry, B Lozzi, K Rajendran, A Cervantes, K Yu, A Jalali, G Rao, SC Mack, B Deneen
Oncogene, 2022-07-15;119(29):e2202015119.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Autophagy and protein aggregation as a mechanism of dopaminergic degeneration in a primary human dopaminergic neuronal model
Authors: E Cuevas, A Guzman, SM Burks, A Ramirez-Le, SF Ali, SZ Imam
Toxicology reports, 2022-04-01;9(0):806-813.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Expanding homogeneous culture of human primordial germ cell-like cells maintaining germline features without serum or feeder layers
Authors: M Kobayashi, M Kobayashi, J Odajima, K Shioda, YS Hwang, K Sasaki, P Chatterjee, C Kramme, RE Kohman, GM Church, AR Loehr, RS Weiss, H Jüppner, JJ Gell, CC Lau, T Shioda
Stem Cell Reports, 2022-02-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids
Authors: AN Cho, Y Jin, Y An, J Kim, YS Choi, JS Lee, J Kim, WY Choi, DJ Koo, W Yu, GE Chang, DY Kim, SH Jo, J Kim, SY Kim, YG Kim, JY Kim, N Choi, E Cheong, YJ Kim, HS Je, HC Kang, SW Cho
Nature Communications, 2021-08-05;12(1):4730.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tanc2-mediated mTOR inhibition balances mTORC1/2 signaling in the developing mouse brain and human neurons
Authors: SG Kim, S Lee, Y Kim, J Park, D Woo, D Kim, Y Li, W Shin, H Kang, C Yook, M Lee, K Kim, JD Roh, J Ryu, H Jung, SM Um, E Yang, H Kim, J Han, WD Heo, E Kim
Nature Communications, 2021-05-11;12(1):2695.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Opposing immune and genetic mechanisms shape oncogenic programs in synovial sarcoma
Authors: L Jerby-Arno, C Neftel, ME Shore, HR Weisman, ND Mathewson, MJ McBride, B Haas, B Izar, A Volorio, G Boulay, L Cironi, AR Richman, LC Broye, JM Gurski, CC Luo, R Mylvaganam, L Nguyen, S Mei, JC Melms, C Georgescu, O Cohen, JE Buendia-Bu, A Segerstolp, M Sud, MS Cuoco, D Labes, S Gritsch, DR Zollinger, N Ortogero, JM Beechem, G Petur Niel, I Chebib, T Nguyen-Ngo, M Montemurro, GM Cote, E Choy, I Letovanec, S Cherix, N Wagle, PK Sorger, AB Haynes, JT Mullen, I Stamenkovi, MN Rivera, C Kadoch, KW Wucherpfen, O Rozenblatt, ML Suvà, N Riggi, A Regev
Nature Medicine, 2021-01-25;0(0):.
Species: Human
Sample Types: Spheroid
Applications: Bioassay -
SLC6A20 transporter: a novel regulator of brain glycine homeostasis and NMDAR function
Authors: M Bae, JD Roh, Y Kim, SS Kim, HM Han, E Yang, H Kang, S Lee, JY Kim, R Kang, H Jung, T Yoo, H Kim, D Kim, H Oh, S Han, D Kim, J Han, YC Bae, H Kim, S Ahn, AM Chan, D Lee, JW Kim, E Kim
Embo Molecular Medicine, 2021-01-11;0(0):e12632.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
SATB2 drives glioblastoma growth by recruiting CBP to promote FOXM1 expression in glioma stem cells
Authors: W Tao, A Zhang, K Zhai, Z Huang, H Huang, W Zhou, Q Huang, X Fang, BC Prager, X Wang, Q Wu, AE Sloan, MS Ahluwalia, JD Lathia, JS Yu, JN Rich, S Bao
EMBO Mol Med, 2020-10-30;0(0):e12291.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
SPT6-driven error-free DNA repair safeguards genomic stability of glioblastoma cancer stem-like cells
Authors: EAA Obara, D Aguilar-Mo, RD Rasmussen, A Frias, K Vitting-Se, YC Lim, KJ Elbæk, H Pedersen, L Vardouli, KE Jensen, J Skjoth-Ras, J Brennum, L Tuckova, R Strauss, C Dinant, J Bartek, P Hamerlik
Nat Commun, 2020-09-18;11(1):4709.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of Functional Brown Adipocytes from Human Pluripotent Stem Cells via Progression through a Paraxial Mesoderm State
Authors: L Zhang, J Avery, A Yin, AM Singh, TS Cliff, H Yin, S Dalton
Cell Stem Cell, 2020-08-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In�Vivo Persistence and Enhances Anti-tumor Activity
Authors: H Zhu, RH Blum, D Bernareggi, EH Ask, Z Wu, HJ Hoel, Z Meng, C Wu, KL Guan, KJ Malmberg, DS Kaufman
Cell Stem Cell, 2020-06-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human beige adipocytes for drug discovery and cell therapy in metabolic diseases
Authors: AM Singh, L Zhang, J Avery, A Yin, Y Du, H Wang, Z Li, H Fu, H Yin, S Dalton
Nat Commun, 2020-06-02;11(1):2758.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Variation of Human Neural Stem Cells Generating Organizer States In�Vitro before Committing to Cortical Excitatory or Inhibitory Neuronal Fates
Authors: N Micali, SK Kim, M Diaz-Busta, G Stein-O'Br, S Seo, JH Shin, BG Rash, S Ma, Y Wang, NA Olivares, JI Arellano, KR Maynard, EJ Fertig, AJ Cross, RW Bürli, NJ Brandon, DR Weinberger, JG Chenoweth, DJ Hoeppner, N Sestan, P Rakic, C Colantuoni, RD McKay
Cell Rep, 2020-05-05;31(5):107599.
Species: Mouse
Sample Types:
Applications: Cell Culture -
Quantitative modelling predicts the impact of DNA methylation on RNA polymerase II traffic
Authors: J Cholewa-Wa, R Shah, S Webb, K Chhatbar, B Ramsahoye, O Pusch, M Yu, P Greulich, B Waclaw, AP Bird
Proc. Natl. Acad. Sci. U.S.A., 2019-07-09;116(30):14995-15000.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Activity of Selected Nucleoside Analogue ProTides against Zika Virus in Human Neural Stem Cells
Authors: JA Bernatchez, M Coste, S Beck, GA Wells, LA Luna, AE Clark, Z Zhu, D Hecht, JN Rich, CD Sohl, BW Purse, JL Siqueira-N
Viruses, 2019-04-20;11(4):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Mechanism of Action of Methotrexate Against Zika Virus
Authors: S Beck, Z Zhu, MF Oliveira, DM Smith, JN Rich, JA Bernatchez, JL Siqueira-N
Viruses, 2019-04-10;11(4):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Human Pluripotent Stem Cell-Derived Multipotent Vascular Progenitors of the Mesothelium Lineage Have Utility in Tissue Engineering and Repair
Authors: T Colunga, M Hayworth, S Kre beta, DM Reynolds, L Chen, KL Nazor, J Baur, AM Singh, JF Loring, M Metzger, S Dalton
Cell Rep, 2019-03-05;26(10):2566-2579.e10.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth
Authors: EK Kim, JH Cho, E Kim, YJ Kim
PLoS ONE, 2017-07-14;12(7):e0181183.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The FDA-approved drug sofosbuvir inhibits Zika virus infection
Authors: Kristen M Bullard-Fe
Antiviral Res, 2016-11-27;137(0):134-140.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FGF2 and insulin signaling converge to regulate cyclin D expression in multipotent neural stem cells.
Authors: Adepoju A, Micali N, Ogawa K, Hoeppner D, McKay R
Stem Cells, 2014-03-01;32(3):770-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling.
Authors: West FD, Machacek DW, Boyd NL, Pandiyan K, Robbins KR, Stice SL
Stem Cells, 2008-08-21;26(11):2768-76.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
What receptors does FGF basic bind?
FGF receptor specificity has been reviewed in multiple citations. Please find more information at: //www.rndsystems.com/resources/articles/fibroblast-growth-factors-and-their-receptors
-
Does human FGF basic show activity on mouse cells?
Yes, it does. The bioassay uses NR-6 mouse fibroblast cells. There is 95% homology between the human and mouse protein and 98% homology between the human and mouse receptor.
Reviews for Recombinant Human FGF basic/FGF2/bFGF, 145 aa TC Grade, CF
Average Rating: 5 (Based on 1 Review)
Have you used Recombinant Human FGF basic/FGF2/bFGF, 145 aa TC Grade, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:



