Recombinant Human/Mouse FGF-8b Protein Summary
Product Specifications
Gln23-Arg215, with an N-terminal Met
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
423-F8
| Formulation | Lyophilized from a 0.2 μm filtered solution in MOPS, Na2SO4 and Brij-35 with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 25 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
423-F8/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in MOPS, Na2SO4 and Brij-35. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
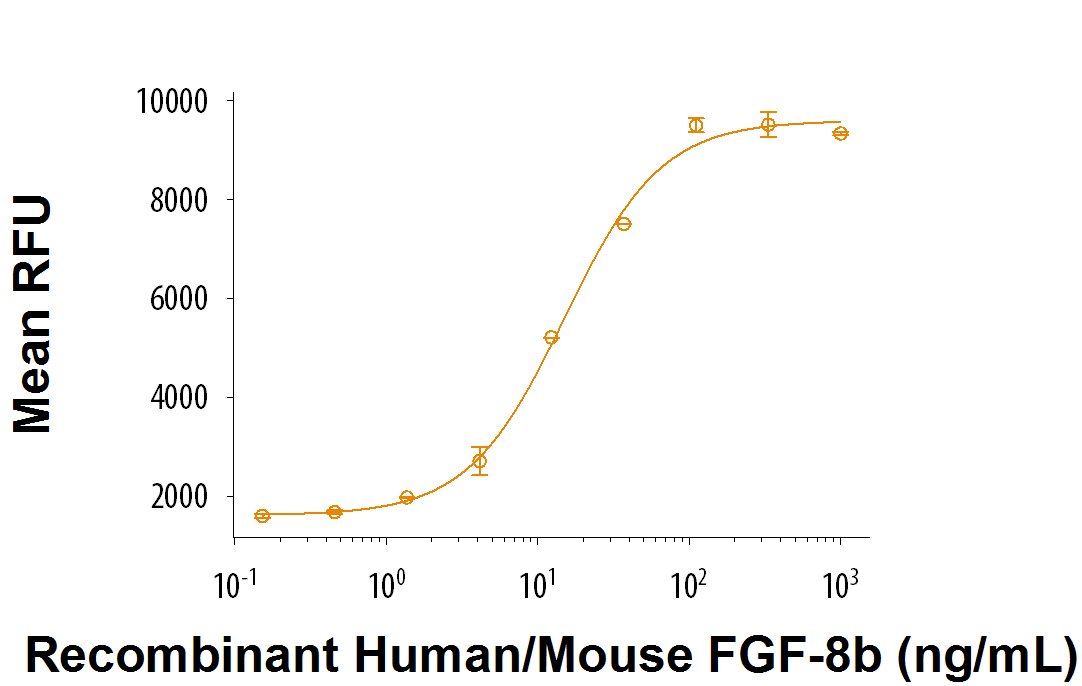
Recombinant Human/Mouse FGF-8b (Catalog # 423-F8) stimulates cell proliferation in the NR6R‑3T3 mouse fibroblast cell line. The ED50 for this effect is 6.5-40 ng/mL in the presence of 1 μg/mL heparin.
 View Larger
View Larger
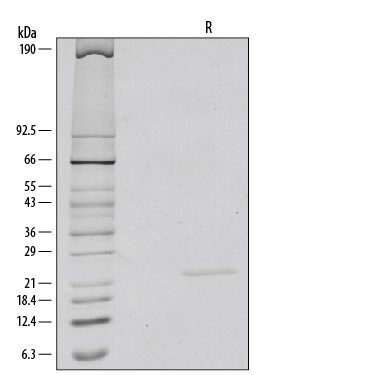
1 μg/lane of Recombinant Human/Mouse FGF-8b was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 23 kDa.
Reconstitution Calculator
Background: FGF-8
FGF-8 is a member of the fibroblast growth factor family that was originally discovered as a growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells (1-3). Alternate splicing of mouse FGF-8 mRNA generates eight secreted isoforms, designated a-h, but only FGF-8a, b, e and f exist in humans (4). FGF-8 contains a 22 amino acid (aa) signal sequence, an N‑terminal domain that varies according to the isoform (30 aa for FGF-8b; 20 aa for the shortest, FGF-8a), a 125 aa FGF domain and a 37 aa proline‑rich C‑terminal sequence. The FGF domain of FGF-8 shares the most aa identity with FGF17 (75%) and FGF-18 (67%), and the three form an FGF subfamily (2). Mouse FGF-8b shares 100% aa identity with human FGF-8b. FGF-8 is widely expressed during embryogenesis, and mediates epithelial-mesenchymal transitions. It plays an organizing and inducing role during gastrulation, and regulates patterning of the midbrain/hindbrain, eye, ear, limbs and heart in the embryo (2, 5 - 8). The isoforms may play different roles in development. FGF-8b shows the strongest receptor affinity and oncogenic transforming capacity although FGF-8a and FGF-8e are also transforming and have been found in human prostate, breast or ovarian tumors (1, 5, 9-12). FGF-8 shows limited expression in the normal adult, but low levels are found in the reproductive and genitourinary tract, peripheral leukocytes and bone marrow hematopoietic cells (3, 9, 13).
- Mattila, M.M. and P.L. Harkonen (2007) Cytokine Growth Factor Rev. 18:257.
- Reuss, B. and O. von Bohlen und Halbach (2003) Cell Tiss. Res. 313:139.
- Tanaka, A. et al. (1992) Proc. Natl. Acad. Sci. USA 89:8928.
- Gemel, J. et al. (1996) Genomics 35:253.
- Olsen, S.K. et al. (2006) Genes Dev. 20:185.
- Crossley, P.H. et al. (1996) Cell, 84:127.
- Heikinheimo, M. et al. (1994) Mech. Dev. 48:129.
- Sun, X. et al. (1999) Genes Dev. 13:1834.
- Ghosh, A.K. et al. (1996) Cell Growth Differ. 7:1425.
- Mattila, M.M. et al. (2001) Oncogene 20:2791.
- Valve, E. et al. (2000) Int. J. Cancer 88:718.
- Valve, E.M. et al. (2001) Lab. Invest. 81:815.
- Nezu, M. et al. (2005) Biochem. Biophys. Res. Commun. 335:843.
Citations for Recombinant Human/Mouse FGF-8b Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
59
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Unconventional secretion of alpha-synuclein mediated by palmitoylated DNAJC5 oligomers
Authors: S Wu, NC Hernandez, DW Sirkis, I Thomas-Wri, R Wade-Marti, R Schekman
Elife, 2023-01-10;12(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A reference human induced pluripotent stem cell line for large-scale collaborative studies
Authors: CB Pantazis, A Yang, E Lara, JA McDonough, C Blauwendra, L Peng, H Oguro, J Kanaujiya, J Zou, D Sebesta, G Pratt, E Cross, J Blockwick, P Buxton, L Kinner-Bib, C Medura, C Tompkins, S Hughes, M Santiana, F Faghri, MA Nalls, D Vitale, S Ballard, YA Qi, DM Ramos, KM Anderson, J Stadler, P Narayan, J Papademetr, L Reilly, MP Nelson, S Aggarwal, LU Rosen, P Kirwan, V Pisupati, SL Coon, SW Scholz, T Priebe, M Öttl, J Dong, M Meijer, LJM Janssen, VS Lourenco, R van der Ka, D Crusius, D Paquet, AC Raulin, G Bu, A Held, BJ Wainger, RMC Gabriele, JM Casey, S Wray, D Abu-Bonsra, CL Parish, MS Beccari, DW Cleveland, E Li, IVL Rose, M Kampmann, C Calatayud, P Verstreken, L Heinrich, MY Chen, B Schüle, D Dou, ELF Holzbaur, MC Zanellati, R Basundra, M Deshmukh, S Cohen, R Khanna, M Raman, ZS Nevin, M Matia, J Van Lent, V Timmerman, BR Conklin, K Johnson Ch, K Zhang, S Funes, DA Bosco, L Erlebach, M Welzer, D Kronenberg, G Lyu, E Arenas, E Coccia, L Sarrafha, T Ahfeldt, JC Marioni, WC Skarnes, MR Cookson, ME Ward, FT Merkle
Cell Stem Cell, 2022-12-01;29(12):1685-1702.e22.
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
Isoform-specific inhibition of FGFR signaling achieved by a de-novo-designed mini-protein
Authors: JS Park, J Choi, L Cao, J Mohanty, Y Suzuki, A Park, D Baker, J Schlessing, S Lee
Cell Reports, 2022-10-25;41(4):111545.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Differentiation of human induced pluripotent stem cells into hypothalamic vasopressin neurons with minimal exogenous signals and partial conversion to the naive state
Authors: H Ozaki, H Suga, M Sakakibara, M Soen, N Miyake, T Miwata, S Taga, T Nagai, M Kano, K Mitsumoto, T Miyata, T Kobayashi, M Sugiyama, T Onoue, H Takagi, D Hagiwara, S Iwama, R Banno, G Iguchi, Y Takahashi, K Muguruma, H Inoue, H Arima
Scientific Reports, 2022-10-17;12(1):17381.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Nuclear Factor I-C Regulates Stemness Genes and Proliferation of Stem Cells in Various Mineralized Tissue through Epithelial-Mesenchymal Interactions in Dental Epithelial Stem Cells
Authors: DS Lee, YJ Song, HR Gug, JH Lee, HS Bae, JC Park
Stem Cells International, 2022-09-27;2022(0):1092184.
Species: Mouse
Sample Types: Whole Cell
Applications: Cell Culture -
Increased Expression and Altered Cellular Localization of Fibroblast Growth Factor Receptor-Like 1 (FGFRL1) Are Associated with Prostate Cancer Progression
Authors: L Yu, M Toriseva, S Afshan, M Cangiano, V Fey, A Erickson, H Seikkula, K Alanen, P Taimen, O Ettala, M Nurmi, PJ Boström, M Kallajoki, J Tuomela, T Mirtti, IJ Beumer, M Nees, P Härkönen
Cancers, 2022-01-07;14(2):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induction of gammadeltaT cells from HSC-enriched BMCs co-cultured with iPSC-derived thymic epithelial cells
Authors: N Hosaka, S Kanda, T Shimono, T Nishiyama
Journal of Cellular and Molecular Medicine, 2021-10-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
3D Bioprinting Mesenchymal Stem Cell-Derived Neural Tissues Using a Fibrin-Based Bioink
Authors: M Restan Per, R Sharma, NZ Masri, SM Willerth
Biomolecules, 2021-08-21;11(8):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FGF8 and BMP2 mediated dynamic regulation of dental mesenchyme proliferation and differentiation via Lhx8/Suv39h1 complex
Authors: C Zhou, D Chen, J Ren, D Huang, R Li, H Luo, C Guan, Y Cao, W Wang
Journal of Cellular and Molecular Medicine, 2021-02-13;0(0):.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Bioassay -
Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use
Authors: TW Kim, J Piao, SY Koo, S Kriks, SY Chung, D Betel, ND Socci, SJ Choi, S Zabierowsk, BN Dubose, EJ Hill, EV Mosharov, S Irion, MJ Tomishima, V Tabar, L Studer
Cell Stem Cell, 2021-02-04;28(2):343-355.e5.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Control of mesenchymal cell fate via application of FGF-8b in vitro
Authors: T Otsuka, PY Mengsteab, CT Laurencin
Stem Cell Research, 2021-01-07;51(0):102155.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Disease-specific phenotypes in iPSC-derived neural stem cells with POLG mutations
Authors: KX Liang, CK Kristianse, S Mostafavi, GH Vatne, GA Zantingh, A Kianian, C Tzoulis, LE Høyland, M Ziegler, RM Perez, J Furriol, Z Zhang, N Balafkan, Y Hong, R Siller, GJ Sullivan, LA Bindoff
EMBO Mol Med, 2020-08-25;0(0):e12146.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Dopamine transporter neuroimaging accurately assesses the maturation of dopamine neurons in a preclinical model of Parkinson's disease
Authors: JL Goggi, L Qiu, MC Liao, S Khanapur, L Jiang, R Boominatha, SV Hartimath, P Cheng, FF Yong, V Soh, X Deng, YM Lin, A Haslop, PW Tan, X Zeng, JWL Lee, Z Zhang, P Sadasivam, EK Tan, SK Luthra, WD Shingleton, SKW Oh, L Zeng, EG Robins
Stem Cell Res Ther, 2020-08-08;11(1):347.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Combined Dendritic and Axonal Deterioration Are Responsible for Motoneuronopathy in Patient-Derived Neuronal Cell Models of Chorea-Acanthocytosis
Authors: H Gla beta, P Neumann, A Pal, P Reinhardt, A Storch, J Sternecker, A Hermann
Int J Mol Sci, 2020-03-05;21(5):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors
Authors: S Mahajani, A Raina, C Fokken, S Kügler, M Bähr
Cell Death Dis, 2019-11-27;10(12):898.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Fibroblast growth factor 8b induces uncoupling protein 1 expression in epididymal white preadipocytes
Authors: S Westphal, T Gantert, C Kless, K Hüttinger, M Klingenspo, T Fromme
Sci Rep, 2019-06-11;9(1):8470.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Modeling Motor Neuron Resilience in ALS Using Stem Cells
Authors: I Allodi, J Nijssen, JA Benitez, C Schweingru, A Fuchs, G Bonvicini, M Cao, O Kiehn, E Hedlund
Stem Cell Reports, 2019-05-09;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Long Non-coding RNAs Associated With Neurodegeneration-Linked Genes Are Reduced in Parkinson's Disease Patients
Authors: M Elkouris, G Kouroupi, A Vourvoukel, N Papagianna, V Kaltezioti, R Matsas, L Stefanis, M Xilouri, PK Politis
Front Cell Neurosci, 2019-02-22;13(0):58.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages
Authors: J Li, F Xing, F Chen, L He, KF So, Y Liu, J Xiao
Cell Transplant, 2018-06-13;0(0):9636897187803.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Alpha6-Containing Nicotinic Acetylcholine Receptors Mediate Nicotine-Induced Structural Plasticity in Mouse and Human iPSC-Derived Dopaminergic Neurons
Authors: G Collo, L Cavalleri, M Zoli, U Maskos, E Ratti, E Merlo Pich
Front Pharmacol, 2018-06-01;9(0):572.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Efficient induction of functional ameloblasts from human keratinocyte stem cells
Authors: X Hu, JW Lee, X Zheng, J Zhang, X Lin, Y Song, B Wang, X Hu, HH Chang, Y Chen, CP Lin, Y Zhang
Stem Cell Res Ther, 2018-05-02;9(1):126.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells
Authors: G Shall, M Menosky, S Decker, P Nethala, R Welchko, X Leveque, M Lu, M Sandstrom, U Hochgeschw, J Rossignol, G Dunbar
Int J Mol Sci, 2018-03-02;19(3):.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Ropinirole and Pramipexole Promote Structural Plasticity in Human iPSC-Derived Dopaminergic Neurons via BDNF and mTOR Signaling
Authors: G Collo, L Cavalleri, F Bono, C Mora, S Fedele, RW Invernizzi, M Gennarelli, G Piovani, T Kunath, MJ Millan, E Merlo Pich, P Spano
Neural Plast., 2018-02-04;2018(0):4196961.
Species: Human
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Chromosomal instability during neurogenesis in Huntington's disease
Authors: A Ruzo, GF Croft, JJ Metzger, S Galgoczi, LJ Gerber, C Pellegrini, H Wang, M Fenner, S Tse, A Marks, C Nchako, AH Brivanlou
Development, 2018-01-29;145(2):.
Species: Human
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Comparative Analysis of Spontaneous and Stimulus-Evoked Calcium Transients in Proliferating and Differentiating Human Midbrain-Derived Stem Cells
Authors: T Johansen, C Krabbe, SI Schmidt, AM Serrano, M Meyer
Stem Cells Int, 2017-10-22;2017(0):9605432.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Integration of Shh and Fgf signaling in controlling Hox gene expression in cultured limb cells
Authors: AR Rodrigues, N Yakushiji-, Y Atsuta, G Andrey, P Schorderet, D Duboule, CJ Tabin
Proc. Natl. Acad. Sci. U.S.A, 2017-03-07;0(0):.
Species: Chicken
Sample Types: Whole Cells
Applications: Bioassay -
Neurotoxic reactive astrocytes are induced by activated microglia
Authors: SA Liddelow, KA Guttenplan, LE Clarke, FC Bennett, CJ Bohlen, L Schirmer, ML Bennett, AE Mnch, WS Chung, TC Peterson, DK Wilton, A Frouin, BA Napier, N Panicker, M Kumar, MS Buckwalter, DH Rowitch, VL Dawson, TM Dawson, B Stevens, BA Barres
Nature, 2017-01-18;541(7638):481-487.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neuronal Dysfunction in iPSC-Derived Medium Spiny Neurons from Chorea-Acanthocytosis Patients Is Reversed by Src Kinase Inhibition and F-Actin Stabilization
Authors: Florian Wegner
J. Neurosci., 2016-11-23;36(47):12027-12043.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Fibulin-1 Binds to Fibroblast Growth Factor 8 with High Affinity: Effects on Embryo Survival
J Biol Chem, 2016-07-08;0(0):.
Species: Human
Sample Types: Protein
Applications: Bioassay -
N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson's Disease: Preliminary Clinical and Cell Line Data
Authors: Daniel A Monti
PLoS ONE, 2016-06-16;11(6):e0157602.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Dopamine Receptor Antagonists Enhance Proliferation and Neurogenesis of Midbrain Lmx1a-expressing Progenitors
Authors: Eva Hedlund
Sci Rep, 2016-06-01;6(0):26448.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
2-O Heparan Sulfate Sulfation by Hs2st Is Required for Erk/Mapk Signalling Activation at the Mid-Gestational Mouse Telencephalic Midline.
Authors: Chan W, Howe K, Clegg J, Guimond S, Price D, Turnbull J, Pratt T
PLoS ONE, 2015-06-15;10(6):e0130147.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Collective cell migration of the nephric duct requires FGF signaling.
Authors: Attia L, Schneider J, Yelin R, Schultheiss T
Dev Dyn, 2014-12-30;244(2):157-67.
Species: Chicken
Sample Types: In Vivo
Applications: Bioassay -
Susceptibility of human embryonic stem cell-derived neural cells to Japanese encephalitis virus infection.
Authors: Shen, Shih-Che, Shen, Ching-I, Lin, Ho, Chen, Chun-Jun, Chang, Chia-Yu, Chen, Sheng-Me, Lee, Hsiu-Chi, Lai, Ping-Sha, Su, Hong-Lin
PLoS ONE, 2014-12-17;9(12):e114990.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Etv1 and Ewsr1 cooperatively regulate limb mesenchymal Fgf10 expression in response to apical ectodermal ridge-derived fibroblast growth factor signal.
Authors: Yamamoto-Shiraishi Y, Higuchi H, Yamamoto S, Hirano M, Kuroiwa A
Dev Biol, 2014-08-07;394(1):181-90.
Species: Chicken
Sample Types: Whole Cells
Applications: Bioassay -
Manipulating gene expression and signaling activity in cultured mouse limb bud cells.
Authors: Lewandowski J, Pursell T, Rabinowitz A, Vokes S
Dev Dyn, 2014-04-17;243(7):928-36.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Transcription factor-induced lineage programming of noradrenaline and motor neurons from embryonic stem cells.
Authors: Mong J, Panman L, Alekseenko Z, Kee N, Stanton L, Ericson J, Perlmann T
Stem Cells, 2014-03-01;32(3):609-22.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Self-assembling peptide nanofiber scaffolds enhance dopaminergic differentiation of mouse pluripotent stem cells in 3-dimensional culture.
Authors: Ni, Na, Hu, Yaohua, Ren, Huixia, Luo, Chuanmin, Li, Peng, Wan, Jian-Bo, Su, Huanxing
PLoS ONE, 2013-12-20;8(12):e84504.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Fgf3 and Fgf10a work in concert to promote maturation of the epibranchial placodes in zebrafish.
Authors: McCarroll, Matthew, Nechiporuk, Alex V
PLoS ONE, 2013-12-17;8(12):e85087.
Species: Zebrafish
Sample Types: Whole Cells
Applications: In Vivo -
MicroRNA-based promotion of human neuronal differentiation and subtype specification.
Authors: Stappert L, Borghese L, Roese-Koerner B, Weinhold S, Koch P, Terstegge S, Uhrberg M, Wernet P, Brustle O
PLoS ONE, 2013-03-18;8(3):e59011.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer.
Authors: Tomlinson DC, Knowles MA
Am. J. Pathol., 2010-10-01;177(5):2379-86.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human embryonic and rat adult stem cells with primitive endoderm-like phenotype can be fated to definitive endoderm, and finally hepatocyte-like cells.
Authors: Roelandt P, Pauwelyn KA, Sancho-Bru P
PLoS ONE, 2010-08-11;5(8):e12101.
Species: Rat
Sample Types: Whole Cells
Applications: Cell Culture -
FGFs, Wnts and BMPs mediate induction of VEGFR-2 (Quek-1) expression during avian somite development.
Authors: Nimmagadda S, Geetha-Loganathan P, Scaal M, Christ B, Huang R
Dev. Biol., 2007-03-01;305(2):421-9.
Species: Avian - Quail
Sample Types: In Vivo
Applications: In Vivo -
Differentiation of ES cells into cerebellar neurons.
Authors: Salero E, Hatten ME
Proc. Natl. Acad. Sci. U.S.A., 2007-02-09;104(8):2997-3002.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue.
Authors: Shimizu T, Bae YK, Hibi M
Development, 2006-11-01;133(23):4709-19.
Species: Zebrafish
Sample Types: Whole Tissue
Applications: Bioassay -
Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm.
Authors: Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM
Development, 2006-08-30;133(19):3787-96.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Expression of the short stature homeobox gene Shox is restricted by proximal and distal signals in chick limb buds and affects the length of skeletal elements.
Authors: Tiecke E, Bangs F, Blaschke R, Farrell ER, Rappold G, Tickle C
Dev. Biol., 2006-07-12;298(2):585-96.
Species: Chicken
Sample Types: In Vivo
Applications: In Vivo -
Embryonic stem cell-derived neuron models of Parkinson's disease exhibit delayed neuronal death.
Authors: Yamashita H, Nakamura T, Takahashi T, Nagano Y, Hiji M, Hirabayashi T, Amano T, Yagi T, Sakai N, Kohriyama T, Matsumoto M
J. Neurochem., 2006-07-01;98(1):45-56.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx.
Authors: Hutson MR, Zhang P, Stadt HA, Sato AK, Li YX, Burch J, Creazzo TL, Kirby ML
Dev. Biol., 2006-06-12;295(2):486-97.
Species: Chicken
Sample Types: Whole Cells
Applications: Bioassay -
Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion.
Authors: Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E
Mol. Cell. Neurosci., 2005-10-21;31(2):251-62.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Control of the segmentation process by graded MAPK/ERK activation in the chick embryo.
Authors: Delfini MC, Dubrulle J, Malapert P, Chal J, Pourquie O
Proc. Natl. Acad. Sci. U.S.A., 2005-07-29;102(32):11343-8.
Species: Chicken
Sample Types: In Vivo
Applications: In Vivo -
Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos.
Authors: Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM
Development, 2005-06-23;132(15):3381-92.
Species: Xenopus
Sample Types: In Vivo
Applications: In Vivo -
Derivation of midbrain dopamine neurons from human embryonic stem cells.
Authors: Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L
Proc. Natl. Acad. Sci. U.S.A., 2004-08-13;101(34):12543-8.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Bioassay -
vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain.
Authors: Wiellette EL, Sive H
Development, 2003-08-01;130(16):3821-9.
Species: Zebrafish
Sample Types: In Vivo
Applications: In Vivo -
Specification of dorsal telencephalic character by sequential Wnt and FGF signaling.
Authors: Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T
Nat. Neurosci., 2003-07-01;6(7):701-7.
Species: Chicken
Sample Types: Whole Tissue
Applications: Bioassay -
Xenopus neurula left-right asymmetry is respeficied by microinjecting TGF-beta5 protein.
Authors: Mogi K, Goto M, Ohno E, Azumi Y, Takeuchi S, Toyoizumi R
Int. J. Dev. Biol., 2003-02-01;47(1):15-29.
Species: Xenopus
Sample Types: In Vivo
Applications: In Vivo -
FGF8 acts as a right determinant during establishment of the left-right axis in the rabbit.
Authors: Fischer A, Viebahn C, Blum M
Curr. Biol., 2002-10-29;12(21):1807-16.
Species: Rabbit
Sample Types: Whole Cells
Applications: Bioassay -
Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse.
Authors: Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN
Development, 2002-10-01;129(19):4613-25.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Regulation of avian cardiogenesis by Fgf8 signaling.
Authors: Alsan BH, 2019, Schultheiss TM
e0007247, 2002-04-01;129(8):1935-43.
Species: Chicken
Sample Types: In Ovo
Applications: In Ovo
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human/Mouse FGF-8b Protein
Average Rating: 5 (Based on 2 Reviews)
Have you used Recombinant Human/Mouse FGF-8b Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
It would be helpful to have larger aliquots.


