Recombinant Human NT-3 Protein
Recombinant Human NT-3 Protein Summary
Product Specifications
Tyr139-Thr257 (K196R)
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
267-N3
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 50 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
267-N3/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute 5 µg vials at 50 µg/mL in sterile PBS. Reconstitute 25 µg or larger vials at 100 µg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
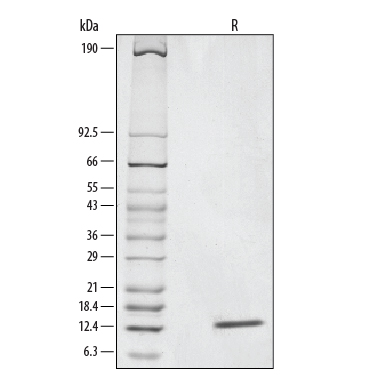 View Larger
View Larger
1 μg/lane of Recombinant Human NT-3 was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 14 kDa.
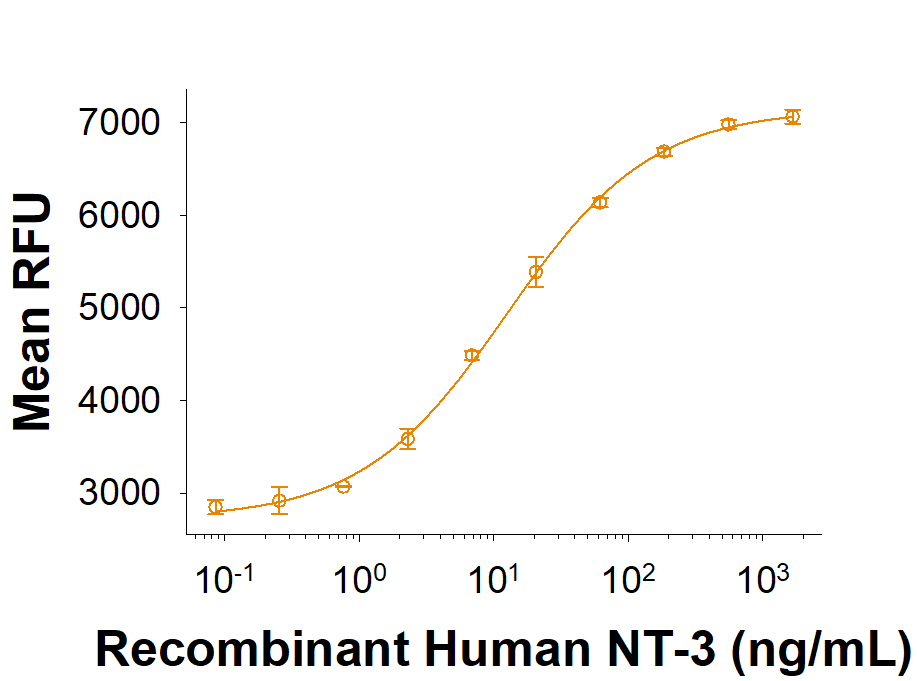 View Larger
View Larger
Recombinant Human NT-3 (Catalog # 267-N3) stimulates cell proliferation in the BaF mouse pro-B cell line transfected with TrkB. The ED50 for this effect is 4.00-40.0 ng/mL.
Reconstitution Calculator
Background: NT-3
Neurotrophin-3 (NT-3) is a member of the NGF family of neurotrophic factors (also named neurotrophins) that are required for the differentiation and survival of specific neuronal subpopulations in both the central as well as the peripheral nervous systems. The neurotrophin family is comprised of at least four proteins including NGF, BDNF, NT-3, and NT-4/5. These secreted cytokines are synthesized as prepropeptides that are proteolytically processed to generate the mature proteins. All neurotrophins have six conserved cysteine residues that are involved in the formation of three disulfide bonds and all share approximately 55% sequence identity at the amino acid level. Similarly to NGF, bioactive NT-3 is predicted to be a non-covalently linked homodimer.
The NT-3 cDNA encodes a 257 amino acid residue precursor protein with a signal peptide and a proprotein that are cleaved to yield the 119 amino acid residue mature NT-3. The amino acid sequence of mature NT-3 is identical in human, mouse and rat. NT-3 transcripts have been detected in the cerebellum, hippocampus, placenta, heart, skin, and skeletal muscle. NT-3 primarily activates the TrkC tyrosine kinase receptor. In addition, NT-3 can activate Trk and TrkB kinase receptors in certain cell systems. NT-3 can also bind with low affinity to the low affinity NGF receptor.
- Eide, F.F. et al. (1993) Exp. Neurol. 121:200.
- Snider, W.D. (1994) Cell 77:627.
- Barbacid, M. (1994) J. Neurobiol. 25:1386.
Citations for Recombinant Human NT-3 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
71
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Neural and metabolic dysregulation in PMM2-deficient human in vitro neural models
Authors: Radenkovic, S;Budhraja, R;Klein-Gunnewiek, T;King, AT;Bhatia, TN;Ligezka, AN;Driesen, K;Shah, R;Ghesquière, B;Pandey, A;Kasri, NN;Sloan, SA;Morava, E;Kozicz, T;
Cell reports
Species: Human hepegivirus
Sample Types: Whole Cells
Applications: Bioassay -
A phenotypic screening platform for identifying chemical modulators of astrocyte reactivity
Authors: Clayton, BLL;Kristell, JD;Allan, KC;Cohn, EF;Karl, M;Jerome, AD;Garrison, E;Maeno-Hikichi, Y;Sturno, AM;Kerr, A;Shick, HE;Sepeda, JA;Freundt, EC;Sas, AR;Segal, BM;Miller, RH;Tesar, PJ;
Nature neuroscience
Species: Human hepegivirus
Sample Types: Whole Cells
Applications: Bioassay -
Preclinical Studies with Glioblastoma Brain Organoid Co-Cultures Show Efficient 5-ALA Photodynamic Therapy
Authors: Pedrosa, L;Bedia, C;Diao, D;Mosteiro, A;Ferr�s, A;Stanzani, E;Mart�nez-Soler, F;Tortosa, A;Pineda, E;Aldecoa, I;Centellas, M;Mu�oz-Tudur�, M;Sevilla, A;Sierra, �;Gonz�lez S�nchez, JJ;
Cells
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Pervasive environmental chemicals impair oligodendrocyte development
Authors: EF Cohn, BLL Clayton, M Madhavan, S Yacoub, Y Federov, K Paul-Fried, TJ Shafer, PJ Tesar
bioRxiv : the preprint server for biology, 2023-02-12;0(0):.
Species: Human, Mouse
Sample Types: Organoid, Whole Cells
Applications: Bioassay -
Stem cell-nanomedicine system as a theranostic bio-gadolinium agent for targeted neutron capture cancer therapy
Authors: YH Lai, CY Su, HW Cheng, CY Chu, LB Jeng, CS Chiang, WC Shyu, SY Chen
Nature Communications, 2023-01-18;14(1):285.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Midkine expression by stem-like tumor cells drives persistence to mTOR inhibition and an immune-suppressive microenvironment
Authors: Y Tang, DJ Kwiatkowsk, EP Henske
Nature Communications, 2022-08-26;13(1):5018.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Oligodendrocyte differentiation alters tRNA modifications and codon optimality-mediated mRNA decay
Authors: S Martin, KC Allan, O Pinkard, T Sweet, PJ Tesar, J Coller
Nature Communications, 2022-08-25;13(1):5003.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cellular analysis of SOD1 protein-aggregation propensity and toxicity: a case of ALS with slow progression harboring homozygous SOD1-D92G mutation
Authors: M Sawamura, K Imamura, R Hikawa, T Enami, A Nagahashi, H Yamakado, H Ichijo, T Fujisawa, H Yamashita, S Minamiyama, M Kaido, H Wada, M Urushitani, H Inoue, N Egawa, R Takahashi
Scientific Reports, 2022-07-25;12(1):12636.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pharmacotherapy alleviates pathological changes in human direct reprogrammed neuronal cell model of myotonic dystrophy type 1
Authors: MK Eltahir, M Nakamori, S Hattori, T Kimura, H Mochizuki, S Nagano
PLoS ONE, 2022-07-01;17(7):e0269683.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Physical and functional interactome atlas of human receptor tyrosine kinases.
Authors: Salokas K, Liu X, Ohman T, Chowdhury I, Gawriyski L, Keskitalo S, Varjosalo M
EMBO Rep, 2022-04-05;23(6):e54041.
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
Pterostilbene in Combination With Mitochondrial Cofactors Improve Mitochondrial Function in Cellular Models of Mitochondrial Diseases
Authors: JM Suárez-Riv, CJ Pastor-Mal, A Romero-Gon, D Gómez-Fern, S Povea-Cabe, M Álvarez-Có, I Villalón-G, M Talaverón-, A Suárez-Car, M Munuera-Ca, JA Sánchez-Al
Frontiers in Pharmacology, 2022-03-18;13(0):862085.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tmem160 contributes to the establishment of discrete nerve injury-induced pain behaviors in male mice
Authors: D Segelcke, HK Fischer, M Hütte, S Dennerlein, F Benseler, N Brose, EM Pogatzki-Z, M Schmidt
Cell Reports, 2021-12-21;37(12):110152.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Reprogramming Human Adult Fibroblasts into GABAergic Interneurons
Authors: A Bruzelius, S Kidnapilla, J Drouin-Oue, T Stoker, RA Barker, D Rylander O
Cells, 2021-12-08;10(12):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TLR4 Associated Signaling Disrupters as a New Means to Overcome HERV-W Envelope-Mediated Myelination Deficits
Authors: P Göttle, K Schichel, L Reiche, L Werner, A Zink, A Prigione, P Küry
Frontiers in Cellular Neuroscience, 2021-11-23;15(0):777542.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Phoenix auditory neurons as 3R cell model for high throughput screening of neurogenic compounds
Authors: F Rousset, D Schmidbaue, S Fink, Y Adel, B Obexer, M Müller, R Glueckert, H Löwenheim, P Senn
Hearing research, 2021-11-14;414(0):108391.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
A microfluidic approach to rescue ALS motor neuron degeneration using rapamycin
Authors: P Chennampal, A Sayed-Zahi, P Soundarara, J Sharp, GA Cox, SD Collins, RL Smith
Scientific Reports, 2021-09-13;11(1):18168.
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CART mitigates oxidative stress and DNA damage in memory deficits of APP/PS1 mice via upregulating &beta?amyloid metabolism?associated enzymes
Authors: H Jiang, F Niu, Y Zheng, Y Xu
Molecular Medicine Reports, 2021-02-19;23(4):1-12.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: CAR-T -
A quantitative model of cellular decision making in direct neuronal reprogramming
Authors: A Merlevede, EM Legault, V Drugge, RA Barker, J Drouin-Oue, V Olariu
Scientific Reports, 2021-01-15;11(1):1514.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Loss of the fragile X syndrome protein FMRP results in misregulation of nonsense-mediated mRNA decay
Authors: T Kurosaki, N Imamachi, C Pröschel, S Mitsutomi, R Nagao, N Akimitsu, LE Maquat
Nature Cell Biology, 2021-01-08;23(1):40-48.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Direct Conversion of Human Stem Cell-Derived Glial Progenitor Cells into GABAergic Interneurons
Authors: J Giacomoni, A Bruzelius, CA Stamouli, D Rylander O
Cells, 2020-11-10;9(11):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Non-canonical Targets of HIF1a Impair Oligodendrocyte Progenitor Cell Function
Authors: KC Allan, LR Hu, MA Scavuzzo, AR Morton, AS Gevorgyan, EF Cohn, BLL Clayton, IR Bederman, S Hung, CF Bartels, M Madhavan, PJ Tesar
Cell Stem Cell, 2020-10-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Direct Reprogramming of Human Fetal- and Stem Cell-Derived Glial Progenitor Cells into Midbrain Dopaminergic Neurons
Authors: S Nolbrant, J Giacomoni, DB Hoban, A Bruzelius, M Birtele, D Chandler-M, M Pereira, DR Ottosson, SA Goldman, M Parmar
Stem Cell Reports, 2020-09-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Modulation of Human Adipose Stem Cells' Neurotrophic Capacity Using a Variety of Growth Factors for Neural Tissue Engineering Applications: Axonal Growth, Transcriptional, and Phosphoproteomic Analyses In Vitro
Authors: KM Prautsch, A Schmidt, V Paradiso, DJ Schaefer, R Guzman, DF Kalbermatt, S Madduri
Cells, 2020-08-21;9(9):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay, Cell Culture -
Age-Dependency of Neurite Outgrowth in Postnatal Mouse Cochlear Spiral Ganglion Explants
Authors: C Frick, S Fink, D Schmidbaue, F Rousset, H Eickhoff, A Tropitzsch, B Kramer, P Senn, R Glueckert, H Rask-Ander, KH Wiesmüller, H Löwenheim, M Müller
Brain Sci, 2020-08-21;10(9):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Maturational Changes in Mouse Cutaneous Touch and Piezo2-Mediated Mechanotransduction
Authors: N Michel, P Narayanan, O Shomroni, M Schmidt
Cell Rep, 2020-07-21;32(3):107912.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Variation of Human Neural Stem Cells Generating Organizer States In�Vitro before Committing to Cortical Excitatory or Inhibitory Neuronal Fates
Authors: N Micali, SK Kim, M Diaz-Busta, G Stein-O'Br, S Seo, JH Shin, BG Rash, S Ma, Y Wang, NA Olivares, JI Arellano, KR Maynard, EJ Fertig, AJ Cross, RW Bürli, NJ Brandon, DR Weinberger, JG Chenoweth, DJ Hoeppner, N Sestan, P Rakic, C Colantuoni, RD McKay
Cell Rep, 2020-05-05;31(5):107599.
Species: Mouse
Sample Types:
Applications: Cell Culture -
Dissecting transcriptomic signatures of neuronal differentiation and maturation using iPSCs
Authors: EE Burke, JG Chenoweth, JH Shin, L Collado-To, SK Kim, N Micali, Y Wang, C Colantuoni, RE Straub, DJ Hoeppner, HY Chen, A Sellers, K Shibbani, GR Hamersky, M Diaz Busta, BN Phan, WS Ulrich, C Valencia, A Jaishankar, AJ Price, A Rajpurohit, SA Semick, RW Bürli, JC Barrow, DJ Hiler, SC Page, K Martinowic, TM Hyde, JE Kleinman, KF Berman, JA Apud, AJ Cross, NJ Brandon, DR Weinberger, BJ Maher, RDG McKay, AE Jaffe
Nat Commun, 2020-01-23;11(1):462.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Transcriptional Programming of Human Mechanosensory Neuron Subtypes from Pluripotent Stem Cells
Authors: AR Nickolls, MM Lee, DF Espinoza, M Szczot, RM Lam, Q Wang, J Beers, J Zou, MQ Nguyen, HJ Solinski, AA AlJanahi, KR Johnson, ME Ward, AT Chesler, CG Bönnemann
Cell Rep, 2020-01-21;30(3):932-946.e7.
Species: Human
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Reprogramming of Fibroblasts to Oligodendrocyte Progenitor-like Cells Using CRISPR/Cas9-Based Synthetic Transcription Factors
Authors: M Matjusaiti, LJ Wagstaff, A Martella, B Baranowski, C Blin, S Gogolok, A Williams, SM Pollard
Stem Cell Reports, 2019-11-07;13(6):1053-1067.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases
Authors: CY Lin, M Yoshida, LT Li, A Ikenaka, S Oshima, K Nakagawa, H Sakurai, E Matsui, T Nakahata, MK Saito
JCI Insight, 2019-09-19;4(18):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A cell fitness selection model for neuronal survival during development
Authors: Y Wang, H Wu, P Fontanet, S Codeluppi, N Akkuratova, C Petitpré, Y Xue-Franzé, K Niederreit, A Sharma, F Da Silva, G Comai, G Agirman, D Palumberi, S Linnarsson, I Adameyko, A Moqrich, A Schedl, G La Manno, S Hadjab, F Lallemend
Nat Commun, 2019-09-12;10(1):4137.
Species: Chicken
Sample Types: Whole Cells
Applications: Cell Culture -
Dysregulated Glial Differentiation in Schizophrenia May Be Relieved by Suppression of SMAD4- and REST-Dependent Signaling
Authors: Z Liu, M Osipovitch, A Benraiss, NPT Huynh, R Foti, J Bates, D Chandler-M, RL Findling, PJ Tesar, M Nedergaard, MS Windrem, SA Goldman
Cell Rep, 2019-06-25;27(13):3832-3843.e6.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis
Authors: D Kremer, J Gruchot, V Weyers, L Oldemeier, P Göttle, L Healy, J Ho Jang, Y Kang T Xu, C Volsko, R Dutta, BD Trapp, H Perron, HP Hartung, P Küry
Proc. Natl. Acad. Sci. U.S.A., 2019-06-18;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Neurotrophin-3 acts on the endothelial-mesenchymal transition of heterotopic ossification in rats
Authors: J Zhang, L Wang, H Cao, N Chen, B Yan, X Ao, H Zhao, J Chu, M Huang, Z Zhang
J. Cell. Mol. Med., 2019-01-22;0(0):.
Species: Rat
Sample Types: In Vivo
Applications: In Vivo -
Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration
Authors: Z Melamed, J López-Erau, MW Baughn, O Zhang, K Drenner, Y Sun, F Freyermuth, MA McMahon, MS Beccari, JW Artates, T Ohkubo, M Rodriguez, N Lin, D Wu, CF Bennett, F Rigo, S Da Cruz, J Ravits, C Lagier-Tou, DW Cleveland
Nat. Neurosci., 2019-01-14;22(2):180-190.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human ESC-Derived Chimeric Mouse Models of Huntington's Disease Reveal Cell-Intrinsic Defects in Glial Progenitor Cell Differentiation
Authors: M Osipovitch, A Asenjo Mar, JN Mariani, A Cornwell, S Dhaliwal, L Zou, D Chandler-M, S Wang, X Li, SJ Benraiss, R Agate, A Lampp, A Benraiss, MS Windrem, SA Goldman
Cell Stem Cell, 2018-12-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A small-molecule inhibitor of SOD1-Derlin-1 interaction ameliorates pathology in an ALS mouse model
Authors: N Tsuburaya, K Homma, T Higuchi, A Balia, H Yamakoshi, N Shibata, S Nakamura, H Nakagawa, SI Ikeda, N Umezawa, N Kato, S Yokoshima, M Shibuya, M Shimonishi, H Kojima, T Okabe, T Nagano, I Naguro, K Imamura, H Inoue, T Fujisawa, H Ichijo
Nat Commun, 2018-07-10;9(1):2668.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Teriflunomide promotes oligodendroglial differentiation and myelination
Authors: P Göttle, A Manousi, D Kremer, L Reiche, HP Hartung, P Küry
J Neuroinflammation, 2018-03-13;15(1):76.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Integrative proteomic and transcriptomic analysis provides evidence for TrkB (NTRK2) as a therapeutic target in combination with tyrosine kinase inhibitors for non-small cell lung cancer
Authors: DR Gomez, LA Byers, M Nilsson, L Diao, J Wang, L Li, P Tong, M Hofstad, B Saigal, I Wistuba, N Kalhor, S Swisher, Y Fan, WK Hong, M Suraokar, C Behrens, C Moran, JV Heymach
Oncotarget, 2018-01-30;9(18):14268-14284.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Alternative NHEJ pathway proteins as components of MYCN oncogenic activity in human neural crest stem cell differentiation: implications for neuroblastoma initiation
Authors: EA Newman, S Chukkapall, D Bashllari, TT Thomas, RA Van Noord, ER Lawlor, MJ Hoenerhoff, AW Opipari, VP Opipari
Cell Death Dis, 2017-12-13;8(12):3208.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Directly Converted Human Fibroblasts Mature to Neurons and Show Long-Term Survival in Adult Rodent Hippocampus
Authors: N Avaliani, U Pfisterer, A Heuer, M Parmar, M Kokaia, M Andersson
Stem Cells Int, 2017-11-26;2017(0):5718608.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Constitutively Active SMAD2/3 Are Broad-Scope Potentiators of Transcription-Factor-Mediated Cellular Reprogramming
Authors: T Ruetz, U Pfisterer, B Di Stefano, J Ashmore, M Beniazza, TV Tian, DF Kaemena, L Tosti, W Tan, JR Manning, E Chantzoura, DR Ottosson, S Collombet, A Johnsson, E Cohen, K Yusa, S Linnarsson, T Graf, M Parmar, K Kaji
Cell Stem Cell, 2017-11-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways
Authors: J Drouin-Oue, S Lau, PL Brattås, D Rylander O, K Pircs, DA Grassi, LM Collins, R Vuono, A Andersson, G Westergren, C Graff, L Minthon, H Toresson, RA Barker, J Jakobsson, M Parmar
EMBO Mol Med, 2017-08-01;9(8):1117-1131.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Comparative Analysis of the Cell Fates of Induced Schwann Cells from Subcutaneous Fat Tissue and Na�ve Schwann Cells in the Sciatic Nerve Injury Model
Authors: M Zhang, MH Jiang, DW Kim, W Ahn, E Chung, Y Son, G Chi
Biomed Res Int, 2017-06-20;2017(0):1252851.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Differentiation of nestin?negative human hair follicle outer root sheath cells into neurons in�vitro
Authors: W Wu, XL Wu, YQ Ji, Z Gao
Mol Med Rep, 2017-05-16;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Novel combinatorial screening identifies neurotrophic factors for selective classes of motor neurons
Authors: S Schaller, D Buttigieg, A Alory, A Jacquier, M Barad, M Merchant, D Gentien, P de la Gran, G Haase
Proc. Natl. Acad. Sci. U.S.A, 2017-03-07;114(12):E2486-E2493.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors
Authors: SK Goparaju, K Kohda, K Ibata, A Soma, Y Nakatake, T Akiyama, S Wakabayash, M Matsushita, M Sakota, H Kimura, M Yuzaki, SB Ko, MS Ko
Sci Rep, 2017-02-13;7(0):42367.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Differentiation of oligodendrocyte progenitor cells from dissociated monolayer and feeder-free cultured pluripotent stem cells
Authors: T Yamashita, Y Miyamoto, Y Bando, T Ono, S Kobayashi, A Doi, T Araki, Y Kato, T Shirakawa, Y Suzuki, J Yamauchi, S Yoshida, N Sato
PLoS ONE, 2017-02-13;12(2):e0171947.
Species: Primate
Sample Types: Whole Cells
Applications: Bioassay -
Arctigenin protects against neuronal hearing loss by promoting neural stem cell survival and differentiation
Authors: Xinghua Huang
Genesis, 2017-02-13;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Small molecules increase direct neural conversion of human fibroblasts
Sci Rep, 2016-12-05;6(0):38290.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Differential gene expression profiles in neurons generated from lymphoblastoid B-cell line-derived iPS cells from monozygotic twin cases with treatment-resistant schizophrenia and discordant responses to clozapine
Authors: T Nakazawa, M Kikuchi, M Ishikawa, H Yamamori, K Nagayasu, T Matsumoto, M Fujimoto, Y Yasuda, M Fujiwara, S Okada, K Matsumura, A Kasai, A Hayata-Tak, N Shintani, S Numata, K Takuma, W Akamatsu, H Okano, A Nakaya, H Hashimoto, R Hashimoto
Schizophr. Res., 2016-10-27;181(0):75-82.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Synapse Formation in Monosynaptic Sensory-Motor Connections Is Regulated by Presynaptic Rho GTPase Cdc42
J Neurosci, 2016-05-25;36(21):5724-35.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Sequential Differentiation of Embryonic Stem Cells into Neural Epithelial-Like Stem Cells and Oligodendrocyte Progenitor Cells
PLoS ONE, 2016-05-18;11(5):e0155227.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Neurotrophin-3 Regulates Synapse Development by Modulating TrkC-PTP? Synaptic Adhesion and Intracellular Signaling Pathways
J Neurosci, 2016-04-27;36(17):4816-31.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
GDF10 is a signal for axonal sprouting and functional recovery after stroke.
Authors: Li S, Nie E, Yin Y, Benowitz L, Tung S, Vinters H, Bahjat F, Stenzel-Poore M, Kawaguchi R, Coppola G, Carmichael S
Nat Neurosci, 2015-10-26;18(12):1737-45.
Species: Human
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Modeling the Early Phenotype at the Neuromuscular Junction of Spinal Muscular Atrophy Using Patient-Derived iPSCs.
Authors: Yoshida M, Kitaoka S, Egawa N, Yamane M, Ikeda R, Tsukita K, Amano N, Watanabe A, Morimoto M, Takahashi J, Hosoi H, Nakahata T, Inoue H, Saito M
Stem Cell Reports, 2015-03-19;4(4):561-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Oligodendroglial maturation is dependent on intracellular protein shuttling.
Authors: Gottle P, Sabo J, Heinen A, Venables G, Torres K, Tzekova N, Parras C, Kremer D, Hartung H, Cate H, Kury P
J Neurosci, 2015-01-21;35(3):906-19.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain.
Authors: Pereira, Maria, Pfisterer, Ulrich, Rylander, Daniella, Torper, Olof, Lau, Shong, Lundblad, Martin, Grealish, Shane, Parmar, Malin
Sci Rep, 2014-09-11;4(0):6330.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The phosphorylation status of Ascl1 is a key determinant of neuronal differentiation and maturation in vivo and in vitro.
Authors: Ali F, Cheng K, Kirwan P, Metcalfe S, Livesey F, Barker R, Philpott A
Development, 2014-05-12;141(11):2216-24.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes.
Authors: Numasawa-Kuroiwa Y, Okada Y, Shibata S, Kishi N, Akamatsu W, Shoji M, Nakanishi A, Oyama M, Osaka H, Inoue K, Takahashi K, Yamanaka S, Kosaki K, Takahashi T, Okano H
Stem Cell Reports, 2014-04-24;2(5):648-61.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reprogramming non-human primate somatic cells into functional neuronal cells by defined factors.
Authors: Zhou, Zhi, Kohda, Kazuhisa, Ibata, Keiji, Kohyama, Jun, Akamatsu, Wado, Yuzaki, Michisuk, Okano, Hirotaka, Sasaki, Erika, Okano, Hideyuki
Mol Brain, 2014-04-03;7(0):24.
Species: Primate - Callitrix jacchus (Common Marmoset)
Sample Types: Whole Cells
Applications: Bioassay -
Canine epidermal neural crest stem cells: characterization and potential as therapy candidate for a large animal model of spinal cord injury.
Authors: Gericota B, Anderson J, Mitchell G, Borjesson D, Sturges B, Nolta J, Sieber-Blum M
Stem Cells Transl Med, 2014-01-17;3(3):334-45.
Species: Canine
Sample Types: Whole Cells
Applications: Bioassay -
Involvement of NT3 and P75(NTR) in photoreceptor degeneration following selective Muller cell ablation.
Authors: Shen W, Zhu L, Lee S, Chung S, Gillies M
J Neuroinflammation, 2013-11-14;10(0):137.
Species: Mouse
Sample Types: In Vivo
-
AAV1.NT-3 gene therapy for charcot-marie-tooth neuropathy.
Authors: Sahenk Z, Galloway G, Clark K, Malik V, Rodino-Klapac L, Kaspar B, Chen L, Braganza C, Montgomery C, Mendell J
Mol Ther, 2013-10-28;22(3):511-21.
Applications: ELISA (Standard) -
Enrichment and characterization of human dermal stem/progenitor cells by intracellular granularity.
Authors: Shim, Joong Hy, Lee, Tae Ryon, Shin, Dong Woo
Stem Cells Dev, 2013-01-22;22(8):1264-74.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Derivation of cerebellar neurons from human pluripotent stem cells.
Authors: Erceg S, Lukovic D, Moreno-Manzano V, Stojkovic M, Bhattacharya S
Curr Protoc Stem Cell Biol, 2012-03-01;20(0):1H.51.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reduced spiral ganglion neuronal loss by adjunctive neurotrophin-3 in experimental pneumococcal meningitis.
Authors: Demel C, Hoegen T, Giese A
J Neuroinflammation, 2011-01-24;8(1):7.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells.
Authors: Delcroix GJ, Curtis KM, Schiller PC, Montero-Menei CN
Differentiation, 2010-09-01;80(4):213-27.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neurotrophins modulate monocyte chemotaxis without affecting macrophage function.
Authors: Samah B, Porcheray F, Gras G
Clin. Exp. Immunol., 2008-01-08;151(3):476-86.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation.
Authors: Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP
Development, 2007-09-01;134(18):3327-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Differentiation of ES cells into cerebellar neurons.
Authors: Salero E, Hatten ME
Proc. Natl. Acad. Sci. U.S.A., 2007-02-09;104(8):2997-3002.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human NT-3 Protein
There are currently no reviews for this product. Be the first to review Recombinant Human NT-3 Protein and earn rewards!
Have you used Recombinant Human NT-3 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image


