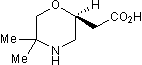
SCH 50911
Chemical Name: (2S)-(+)-5,5-Dimethyl-2-morpholineacetic acid
Purity: ≥98%
Biological Activity
SCH 50911 is a selective, competitive and orally active GABAB antagonist. Displays an IC50 of 1.1 μM at GABAB, approximately 60 times that of CGP 35348 (Cat. No. 1245) and no binding affinity for GABAA at concentrations up to 100 μM.Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Additional Information
Background References
-
The pharmacology of SCH 50911: a novel, orally active GABA-B receptor antagonist.
Bolser et al.
J.Pharmacol.Exp.Ther., 1995;274:1393 -
Characterization of the antiabsence effects of SCH 50911, a GABAB receptor antagonist, in the lethargic mouse, γ-hydroxybutyrate, and pentylenetetrazole models.
Hosford et al.
J.Pharmacol. Exp.Ther., 1995;274:1399 -
The morpholino-acetic acid analogue Sch 50911 is a selective GABAB receptor antagonist in rat neocortical slices.
Ong et al.
Eur.J.Pharmacol., 1998;362:35 -
Substituted morpholine-2S-acetic acid derivatives: SCH 50911 and related compounds as novel GABAB antagonists.
Blythin et al.
Bioorg.Med.Chem.Lett., 1996;6:1529
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for SCH 50911
The citations listed below are publications that use Tocris products. Selected citations for SCH 50911 include:
10 Citations: Showing 1 - 10
-
Dentate cannabinoid-sensitive interneurons undergo unique and selective strengthening of mutual synaptic inhibition in experimental epilepsy.
Authors: Yu Et al.
Neurobiol Dis 2016;89:23
-
Human cerebrospinal fluid monoclonal N-MthD.-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis.
Authors: Kreye Et al.
Brain 2016;139:2641
-
Reduction in focal ictal activity following transplantation of MGE interneurons requires expression of the GABAA receptor α4 subunit.
Authors: Jaiswal Et al.
J Psychopharmacol 2015;9:127
-
The reinforcing effects of ethanol within the nucleus accumbens shell involve activation of local GABA and serotonin receptors.
Authors: Ding Et al.
Br J Pharmacol 2015;29:725
-
Prediction and validation of a mechanism to control the threshold for inhibitory synaptic plasticity.
Authors: Kitagawa Et al.
Mol Syst Biol 2009;5:280
-
Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis.
Authors: Fritsch Et al.
Neuroscience 2009;163:415
-
Evidence for low GluR2 AMPA receptor subunit expression at synapses in the rat basolateral amygdala.
Authors: Gryder Et al.
Toxins (Basel) 2005;94:1728
-
Ligand-induced signal transduction within heterodimeric GABA(B) receptor.
Authors: Margeta-Mitrovic Et al.
Front Cell Neurosci 2001;98:14643
-
The human GABA(B1b) and GABA(B2) heterodimeric recombinant receptor shows low sensitivity to phaclofen and saclofen.
Authors: Wood Et al.
J Neurochem 2000;131:1050
-
Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus.
Authors: Vogt and Nicoll
Proc Natl Acad Sci U S A 1999;96:1118
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for SCH 50911
There are currently no reviews for this product. Be the first to review SCH 50911 and earn rewards!
Have you used SCH 50911?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image