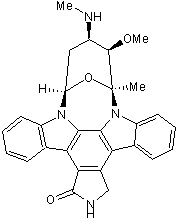
Staurosporine
Chemical Name: [9S-(9α,10β,11β,13α)]-2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one
Purity: ≥98%
Biological Activity
Staurosporine is a broad spectrum protein kinase inhibitor. Enzymes inhibited include protein kinase C, p60v-src tyrosine protein kinase, protein kinase A, and CaM kinase II (IC50 values are 3nM, 6 nM, 7 nM and 20 nM, respectively). Staurosporine reduces nuclear myosin heavy chain 9 phosphorylation which inhibits gastric cancer cell progression in transgenic mouse models. Staurosporine inhibits cell viability and promotes apoptosis in oral and pancreatic cancer cells. Staurosporine also enhances efficiency of lentiviral transduction of human hematopoietic stem and progenitor cells by 2-fold, induces dopaminergic axonal outgrowth in vitro and triggers mitophagy. Staurosporine has high affinity (Kd = 100 nM) for the yjdF aptamer. The riboswitch function of yjdF motif RNAs is activated by Staurosporine and leads to robust reporter gene expressions in B. subtilis.Staurosporine synthesized to Ancillary Material Grade is also available.
Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Background References
-
Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase.
Tamaoki et al.
Biochem.Biophys.Res.Commun., 1986;135:397 -
Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases.
Ruegg and Burgess
TiPS, 1989;10:218 -
Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II.
Yanagihara et al.
J.Neurochem., 1991;56:294 -
Staurosporine increases lentiviral vector transduction efficiency of human hematopoietic stem and progenitor cells.
Lewis et al.
Mol.Ther.Methods Clin.Dev., 2018;9:313
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for Staurosporine
The citations listed below are publications that use Tocris products. Selected citations for Staurosporine include:
20 Citations: Showing 1 - 10
-
High accuracy label-free classification of single-cell kinetic states from holographic cytometry of human melanoma cells.
Authors: Hejna Et al.
Sci Rep 2017;7:11943
-
Matrine induces RIP3-dependent necroptosis in cholangiocarcinoma cells.
Authors: Xu Et al.
Cell Death Discov 2017;3:16096
-
The microanatomic segregation of selection by apoptosis in the germinal center.
Authors: Mayer Et al.
Science 2017;358
-
mTORC1 Couples Nucleotide Synthesis to Nucleotide Demand Resulting in a Targetable Metabolic Vulnerability.
Authors: Valvezan Et al.
Cancer Cell 2017;32:624
-
Integrative genomic and functional analysis of human oral squamous cell carcinoma cell lines reveals synergistic effects of FAT1 and CASP8 inactivation.
Authors: Hayes Et al.
Cancer Lett 2016;383:106
-
Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS.
Authors: Park Et al.
Nat Immunol 2016;17:914
-
Androgen-Sensitized Apoptosis of HPr-1AR Human Prostate Epithelial Cells.
Authors: Chen Et al.
Mol Pharmacol 2016;11:e0156145
-
Identification of a DYRK1A Inhibitor that Induces Degradation of the Target Kinase using Co-chaperone CDC37 fused with Luciferase nanoKAZ.
Authors: Sonamoto Et al.
Eneuro 2015;5:12728
-
Neurotensin Induces Presynaptic Depression of D2 DA Autoreceptor-Mediated Neurotransmission in Midbrain DArgic Neurons.
Authors: Piccart Et al.
J Neurosci 2015;35:11144
-
FMRP regulates neurogenesis in vivo in Xenopus laevis tadpoles.
Authors: Faulkner Et al.
PLoS One 2015;2:e0055
-
IF. gamma induces protective non-canonical signaling pathways in primary neurons.
Authors: O'Donnell Et al.
J Neurochem 2015;135:309
-
Caffeine Modulates Vesicle Release and Recovery at Cerebellar Parallel Fibre Terminals, Independently of Calcium and Cyclic AMP Signalling.
Authors: Dobson Et al.
Eur J Neurosci 2015;10:e0125974
-
Small molecule screen yields inhibitors of Pseudomonas homoserine lactone-induced host responses.
Authors: Valentine Et al.
Cell Microbiol 2014;16:1
-
Enzymatic activity of CaMKII is not required for its interaction with the glutamate receptor subunit GluN2B.
Authors: Barcomb Et al.
J Biol Chem 2013;84:834
-
Development of highly potent and selective diaminothiazole inhibitors of cyclin-dependent kinases.
Authors: Schönbrunn Et al.
J Med Chem 2013;56:3768
-
Pharmacological inhibition of pleckstrin homology domain leucine-rich repeat protein phosphatase is neuroprotective: differential effects on astrocytes.
Authors: Jackson Et al.
J Pharmacol Exp Ther 2013;347:516
-
Effect of activation of canonical Wnt signaling by the Wnt-3a protein on the susceptibility of PC12 cells to oxidative and apoptotic insults.
Authors: Kawamoto Et al.
PLoS One 2012;45:58
-
Contractile effect of tachykinins on rabbit small intestine.
Authors: Valero Et al.
Acta Pharmacol Sin 2011;32:487
-
Poly(ADP-ribose) polymerase (PARP)-1-independent apoptosis-inducing factor (AIF) release and cell death are induced by eleostearic acid and blocked by α-tocopherol and MEK inhibition.
Authors: Kondo Et al.
Braz J Med Biol Res 2010;285:13079
-
N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury.
Authors: Chen Et al.
J Biol Chem 2006;281:2764
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for Staurosporine
There are currently no reviews for this product. Be the first to review Staurosporine and earn rewards!
Have you used Staurosporine?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image