StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL
StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL Summary
Media supplement to induce the differentiation of mouse or rat MSCs into osteocytes. For use with Human/Mouse/Rat StemXVivo® Osteogenic/Adipogenic Base Media (Catalog # CCM007).
Key Benefits
- Supports induction of osteogenesis in mouse and rat MSCs
- Defined supplement to reduce experimental variation
- Developed and optimized using mouse and rat MSCs
Why Induce Osteogenesis in MSCs with a Defined Media Supplement?
Despite the well-characterized factors and protocols used to differentiate mesenchymal stem/stromal cells (MSCs) into osteocytes, differentiation efficiencies can vary depending on the quality of the MSC starting population and the reagents used to expand and differentiate MSCs.
StemXVivo® Osteogenic Supplement:
- Contains high quality differentiation factors to drive reproducible and efficient MSC osteogenesis.
- Is defined to reduce unwanted experimental variability.
- Has been developed and optimized using MSCs.
The term ‘mesenchymal stromal cells’ is commonly used to describe a heterogeneous population of cultured cells that are adherent to plastic, have a distinct morphology, and express a specific set of marker proteins. Within this heterogeneous population are cells referred to as ‘mesenchymal stem cells.’
Mesenchymal stem cells are multipotent, self-renewing cells that have the ability to differentiate into adipocytes, chondrocytes, and osteoblasts when cultured in vitro. Read More about MSC Nomenclature
Mouse/Rat StemXVivo® Osteogenic Supplement Components
This media supplement contains high quality factors to drive mouse/rat MSC differentiation into osteocytes.
- This supplement requires media (not included), such as Human/Mouse/Rat StemXVivo® Osteogenic/Adipogenic Base Media (Catalog # CCM007) or equivalent.
- The quantity of osteogenic media supplement supplied is sufficient to make 250 mL of media for differentiation.
2006 Proposed Change to MSC Nomenclature
Although mesenchymal stromal cells were once referred to as ‘mesenchymal stem cells’, a change to ‘mesenchymal stromal cells’ was proposed by the International Society for Cellular Therapy in 2006.1
The change in nomenclature originates from two important factors:
- Methods used to isolate mesenchymal stem cells yield a heterogeneous population of cells with only a fraction of these cells demonstrating multipotency.
- The absence of direct evidence that mesenchymal stem cells can self-renew and differentiate in vivo.
Use of Mesenchymal Stem and Stromal Cell Terminology
Data supporting MSC self-renewal and multipotency have been obtained using in vitro conditions, which does not adequately reflect the in vivo environment. The lack of in vivo data has led some researchers to question the validity of the term ‘mesenchymal stem cell’ providing further support for the use of ‘mesenchymal stromal cells’ to describe MSCs.2 While ‘mesenchymal stromal cells’ may be the more scientifically accurate term for MSCs, the two terms are often used interchangeably in the literature. R&D Systems recognizes the use of both mesenchymal stem cells and mesenchymal stromal cells and uses ‘MSC’ to indicate mesenchymal stem/stromal cells to account for both designations.
Definitions of Mesenchymal Stromal Cells and Mesenchymal Stem Cells
- Mesenchymal Stromal Cells – A heterogeneous population of cultured cells with similar characteristics such as the ability to adhere to plastic and the expression of specific marker proteins.
- Mesenchymal Stem Cells – A subpopulation of mesenchymal stromal cells that have the capacity to self-renew and differentiate into mesodermal lineages when cultured in vitro. The capacity to self-renew and differentiate in vivo has yet to be clearly demonstrated for mesenchymal stem cells.
References
- Dominici, M. et al. (2006) Cytotherapy 8:315.
- Keating, A. (2012) Cell Stem Cell 10:709.
Specifications
Product Datasheets
Scientific Data
 View Larger
View Larger
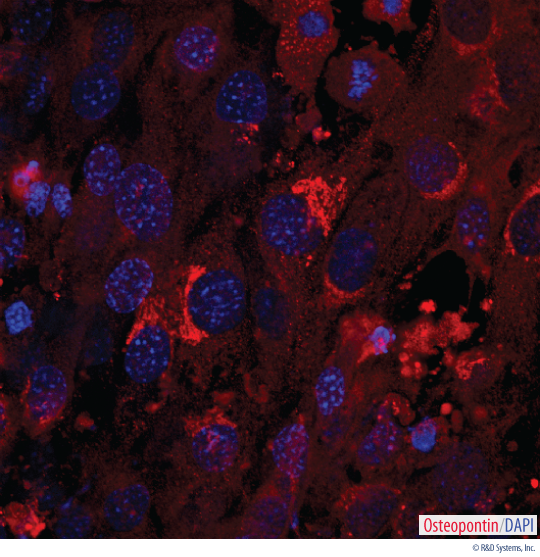
Detection of Osteopontin in Mouse MSC-differentiated Osteocytes. Mouse MSCs were cultured for 14 days using the Human/Mouse/Rat StemXVivo®Osteogenic/Adipogenic Base Media (Catalog # CCM007) and Mouse/Rat StemXVivo®Osteogenic Supplement (Catalog # CCM009). Osteocyte differentiation was verified using a Goat Anti-Mouse Osteopontin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF808). The cells were stained using a NorthernLights™557-conjugated Donkey Anti-Goat Secondary Antibody (Catalog # NL010) and the nuclei were stained with DAPI.
 View Larger
View Larger
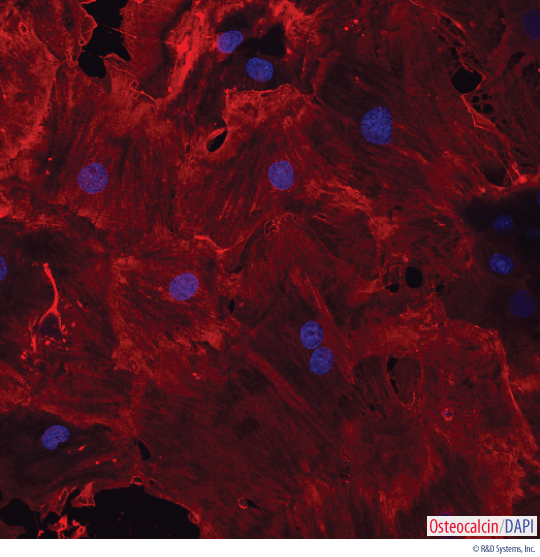
Detection of Osteocalcin in Rat MSC-differentiated Osteocytes. Rat MSCs were cultured for 14 days using the Human/Mouse/Rat StemXVivo®Osteogenic/Adipogenic Base Media (Catalog # CCM007) and Mouse/Rat StemXVivo®Osteogenic Supplement (Catalog # CCM009). Osteocyte differentiation was verified using a Mouse Anti-Human Osteocalcin Monoclonal Antibody (Catalog # MAB1419). The cells were stained using a NorthernLights™557-conjugated Donkey Anti-Mouse Secondary Antibody (Catalog # NL010; red) and the nuclei were counterstained with DAPI (blue).
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, mouse or rat MSCs are differentiated into osteocytes using the following in vitro differentiation procedure:
- Culture multipotent cells of interest
- Induce osteogenic differentiation using a media supplement
- Evaluate differentiation using a mature phenotype marker antibody and fluorescent ICC
For use with Human/Mouse/Rat StemXVivo® Osteogenic/Adipogenic Base Media (Catalog # CCM007).
Reagents supplied in the Mouse/Rat StemXVivo® Osteogenic Supplement (Catalog # CCM009):
- 12.5 mL of StemXVivo® Osteogenic Supplement
Note: The quantity of osteogenic media supplement supplied is sufficient to make 250 mL of media for differentiation.
Reagents
- StemXVivo® Osteogenic/Adipogenic Base Media (Catalog # CCM007 or equivalent)
- Penicillin-Streptomycin-Glutamate (100X)
Materials
- Mouse or rat MSCs
- 10 cm tissue culture plates
- 15 mL centrifuge tubes
- Pipettes and pipette tips
- Serological pipettes
Equipment
- 37 °C and 5% CO2 incubator
- Centrifuge
- Hemocytometer
- Inverted microscope
- 2 °C to 8 °C refrigerator
- 37 °C water bath
This protocol has been tested using bone marrow- and/or adipose tissue-derived MSCs. If using a different tissue source or cell line, the protocol below may need to be optimized.
Osteogenic Differentiation
Plate 4.2 x 103 MSCs/cm2 in StemXVivo® Osteogenic/Adipogenic Base Media.
Culture cells to 50-70% confluency.

Replace the medium with StemXVivo® Osteogenic Differentiation Medium to induce osteogenesis.

Every 3-4 days, replace with fresh Osteogenic Differentiation Medium.
After 14 days, osteocytes can be harvested and analyzed.

Citations for StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
16
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A neomorphic variant in SP7 alters sequence specificity and causes a high-turnover bone disorder
Authors: JC Lui, A Raimann, H Hojo, L Dong, P Roschger, B Kikani, U Wintergers, N Fratzl-Zel, YH Jee, G Haeusler, J Baron
Nature Communications, 2022-02-04;13(1):700. 2022-02-04
-
Regional specialization and fate specification of bone stromal cells in skeletal development
Authors: KK Sivaraj, HW Jeong, B Dharmaling, D Zeuschner, S Adams, M Potente, RH Adams
Cell Reports, 2021-07-13;36(2):109352. 2021-07-13
-
TNF&alpha/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function
Authors: G Beldi, M Khosravi, ME Abdelgawad, BL Salomon, G Uzan, H Haouas, S Naserian
Stem Cell Res Ther, 2020-07-16;11(1):281. 2020-07-16
-
Murine Mesenchymal Stromal Cells Retain Biased Differentiation Plasticity Towards Their Tissue of Origin
Authors: TT Ng, KH Mak, C Popp, RK Ng
Cells, 2020-03-19;9(3):. 2020-03-19
-
Identification of Proteins Differentially Expressed by Adipose-derived Mesenchymal Stem Cells Isolated from Immunodeficient Mice
Authors: Y Nakashima, S Nahar, C Miyagi-Shi, T Kinjo, N Kobayashi, S Kitamura, I Saitoh, M Watanabe, J Fujita, H Noguchi
Int J Mol Sci, 2019-05-30;20(11):. 2019-05-30
-
Metabolic Phenotyping of Adipose-Derived Stem Cells Reveals a Unique Signature and Intrinsic Differences between Fat Pads
Authors: C Lefevre, B Panthu, D Naville, S Guibert, C Pinteur, B Elena-Herr, H Vidal, GJP Rautureau, A Mey
Stem Cells Int, 2019-05-14;2019(0):9323864. 2019-05-14
-
Long-term regeneration and remodeling of the pig esophagus after circumferential resection using a retrievable synthetic scaffold carrying autologous cells
Authors: S La Frances, JM Aho, MR Barron, EW Blanco, S Soliman, L Kalenjian, AD Hanson, E Todorova, M Marsh, K Burnette, H DerSimonia, RD Odze, DA Wigle
Sci Rep, 2018-03-07;8(1):4123. 2018-03-07
-
Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice
Authors: KL Hayes, LM Messina, LM Schwartz, J Yan, AS Burnside, S Witkowski
Am. J. Physiol., Cell Physiol., 2018-01-10;0(0):. 2018-01-10
-
Matrix metalloproteinase processing of PTHrP yields a selective regulator of osteogenesis, PTHrP1-17
Authors: JS Frieling, G Shay, V Izumi, ST Aherne, RG Saul, M Budzevich, J Koomen, CC Lynch
Oncogene, 2017-04-03;0(0):. 2017-04-03
-
Purification and differentiation of human adipose-derived stem cells by membrane filtration and membrane migration methods
Authors: HR Lin, CW Heish, CH Liu, S Muduli, HF Li, A Higuchi, SS Kumar, AA Alarfaj, MA Munusamy, ST Hsu, DC Chen, G Benelli, K Murugan, NC Cheng, HC Wang, GJ Wu
Sci Rep, 2017-01-10;7(0):40069. 2017-01-10
-
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
Authors: Ramasamy S, Kusumbe A, Wang L, Adams R
Nature, 2014-03-12;507(7492):376-80. 2014-03-12
-
Purification of human adipose-derived stem cells from fat tissues using PLGA/silk screen hybrid membranes.
Authors: Chen D, Chen L, Ling Q, Wu M, Wang C, Suresh Kumar S, Chang Y, Munusamy M, Alarfajj A, Wang H, Hsu S, Higuchi A
Biomaterials, 2014-02-22;35(14):4278-87. 2014-02-22
-
Label retention identifies a multipotent mesenchymal stem cell-like population in the postnatal thymus.
Authors: Osada M, Singh V, Wu K, Sant'Angelo D, Pezzano M
PLoS ONE, 2013-12-10;8(12):e83024. 2013-12-10
-
Molecular characterization of prospectively isolated multipotent mesenchymal progenitors provides new insight into the cellular identity of mesenchymal stem cells in mouse bone marrow.
Authors: Qian H, Badaloni A, Chiara F, Stjernberg J, Polisetti N, Nihlberg K, Consalez G, Sigvardsson M
Mol Cell Biol, 2012-11-26;33(4):661-77. 2012-11-26
-
Cellular basis of tissue regeneration by omentum.
Authors: Shah S, Lowery E, Braun RK
PLoS ONE, 2012-06-06;7(6):e38368. 2012-06-06
-
3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells.
Authors: Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI, Yamasaki Y, Kataoka K
Biomaterials, 2009-02-12;30(14):2705-15. 2009-02-12
FAQs
No product specific FAQs exist for this product, however you may
View all Cell Culture Product FAQsReviews for StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL
There are currently no reviews for this product. Be the first to review StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL and earn rewards!
Have you used StemXVivo Mouse/Rat Osteogenic Supplement, 12.5 mL?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

