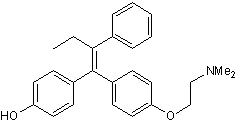
(Z)-4-Hydroxytamoxifen
Chemical Name: 4-[(1Z)-1-[4-[2-(Dimethylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]phenol
Purity: ≥98%
Biological Activity
(Z)-4-Hydroxytamoxifen is an estrogen receptor antagonist (IC50 = 27 μM). (Z)-4-Hydroxytamoxifen is a Tamoxifen (Cat. No. 0999) metabolite; that exhibits greater potency than the parent compound. (Z)-4-Hydroxytamoxifen also activates intein-linked inactive Cas9, reducing off-target CRISPR-mediated gene editing; system has ~25-fold higher specificity than wtCas9. (Z)-4-Hydroxytamoxifen binds to voltage-gated sodium channels, near the intracellular gate and inhibits the sodium current by delaying channel recovery from the inactivated state (IC50 values are 297 nM and 2.1 �M for NavM and human Nav, respectively in HEK cells); this inhibition is independent of estrogen receptor modulation. Chemotherapeutic agent.Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Background References
-
Simple and efficient production of (Z)-4-hydroxytamoxifen, a potent estrogen receptor modulator.
Yu and Forman
J.Org.Chem., 2003;68:9489 -
Small molecule-triggered Cas9 protein with improved genome-editing specificity.
Davis et al.
Nat.Chem.Biol., 2015;11:316 -
A monohydroxylated metabolite of tamox. with potent antioestrogenic activity.
Jordan et al.
J.Endocrinology, 1977;75:305 -
Comprehensive evaluation of tamox. sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6.
Desta et al.
J.Pharm.Exp.Ther., 2004;310:1062
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for (Z)-4-Hydroxytamoxifen
The citations listed below are publications that use Tocris products. Selected citations for (Z)-4-Hydroxytamoxifen include:
5 Citations: Showing 1 - 5
-
The BAF and PRC2 Complex Subunits Dpf2 and Eed Antagonistically Converge on Tbx3 to Control ESC Differentiation.
Authors: Zhang Et al.
Cell Stem Cell 2019;24:138
-
Paracrine Crosstalk between Fibroblasts and ER+ Breast Cancer Cells Creates an IL1β-Enriched Niche that Promotes Tumor Growth.
Authors: Chatterjee Et al.
iScience 2019;19:388
-
PTK6 regulates growth and survival of endocrine therapy-resistant ER+ breast cancer cells.
Authors: Ito Et al.
NPJ Breast Cancer 2017;3:45
-
MTA1 is a novel regulator of autophagy that induces TAX resistance in breast cancer cells.
Authors: Lee Et al.
Autophagy 2017;14:812
-
Alcohol Regulates Genes that Are Associated with Response to Endocrine Therapy and Attenuates the Actions of tamox. in Breast Cancer Cells.
Authors: Candelaria Et al.
PLoS One 2015;10:e0145061
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for (Z)-4-Hydroxytamoxifen
There are currently no reviews for this product. Be the first to review (Z)-4-Hydroxytamoxifen and earn rewards!
Have you used (Z)-4-Hydroxytamoxifen?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image