Human IL-12/IL-23 p40 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-12/IL-23 p40. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
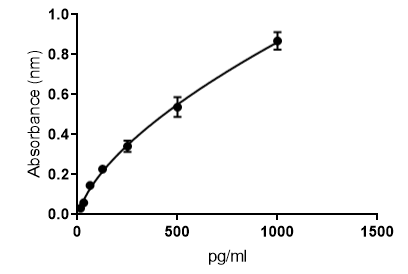
Scientific Data
Product Datasheets
Preparation and Storage
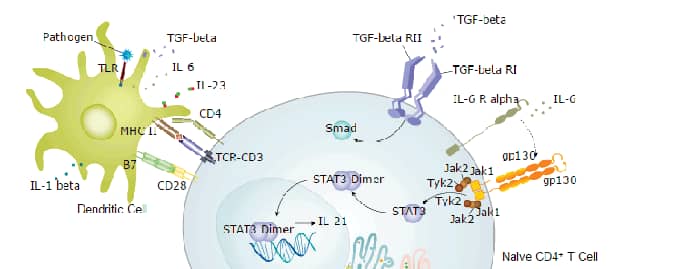
Background: IL-12/IL-23 p40
Interleukin 12 (IL-12), also known as natural killer cell stimulatory factor (NKSF) or cytotoxic lymphocyte maturation factor (CLMF), is a heterodimeric pleiotropic cytokine made up of a 40 kDa (p40) subunit and a 35 kDa (p35) subunit. The IL-12 p40 subunit is shared by IL-23, another heterodimeric cytokine that has biological activities similar to, as well as distinct from, IL-12. IL-12 is produced by macrophages and B cells and has been shown to have multiple effects on T cells and natural killer (NK) cells. While mouse IL-12 is active on both human and mouse cells, human IL-12 is not active on mouse cells.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent with NGS, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-12/IL-23 p40 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
32
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Nuclear factor kappa B-dependent persistence of Salmonella Typhi and Paratyphi in human macrophages
Authors: Stepien, TA;Singletary, LA;Guerra, FE;Karlinsey, JE;Libby, SJ;Jaslow, SL;Gaggioli, MR;Gibbs, KD;Ko, DC;Brehm, MA;Greiner, DL;Shultz, LD;Fang, FC;
mBio
Species: Human hepegivirus
Sample Types: Cell Culture Supernates
-
Asprosin Exerts Pro-Inflammatory Effects in THP-1 Macrophages Mediated via the Toll-like Receptor 4 (TLR4) Pathway
Authors: K Shabir, S Gharanei, S Orton, V Patel, P Chauhan, E Karteris, HS Randeva, JE Brown, I Kyrou
International Journal of Molecular Sciences, 2022-12-23;24(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
Authors: LAJ Feather, V Nadella, E Kastner, Y Perrie, AC Hilton, A Devitt
Scientific Reports, 2022-07-20;12(1):12448.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of sulfated secondary bile acids on intestinal barrier function and immune response in an inflammatory in vitro human intestinal model
Authors: B van der Lu, MCP Vos, M Grootte Br, N Ijssennagg, F Vrieling, J Meijerink, WT Steegenga
Heliyon, 2022-02-02;8(2):e08883.
Species: Human
Sample Types: Cell Culture Supernates
-
Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit
Authors: T Katopodi, S Petanidis, K Domvri, P Zarogoulid, D Anestakis, C Charalampi, D Tsavlis, C Bai, H Huang, L Freitag, W Hohenforst, D Matthaios, K Porpodis
Scientific Reports, 2021-07-29;11(1):15455.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel immunomodulatory properties of low dose cytarabine entrapped in a mannosylated cationic liposome
Authors: AL Martel, NL Fraleigh, E Picard, JD Lewicky, G Pawelec, H Lee, GW Ma, L Mousavifar, R Roy, HT Le
International journal of pharmaceutics, 2021-07-01;0(0):120849.
Species: Human
Sample Types: Cell Culture Supernates
-
Newly defined ABCB5+ dermal mesenchymal stem cells promote healing of chronic iron overload wounds via secretion of interleukin-1 receptor antagonist
Authors: S Vander Bek, JC de Vries, B Meier-Schi, P Meyer, D Jiang, A Sindrilaru, FF Ferreira, A Hainzl, S Schatz, J Muschhamme, NJ Scheurmann, P Kampilafko, AM Seitz, L Dürselen, A Ignatius, MA Kluth, C Ganss, M Wlaschek, K Singh, P Maity, NY Frank, MH Frank, K Scharffett
Stem Cells, 2019-05-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Authors: C Wei, C Yang, S Wang, D Shi, C Zhang, X Lin, Q Liu, R Dou, B Xiong
Mol. Cancer, 2019-03-30;18(1):64.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasminogen Activator Inhibitor-1 Promotes the Recruitment and Polarization of Macrophages in Cancer
Authors: MH Kubala, V Punj, VR Placencio-, H Fang, GE Fernandez, R Sposto, YA DeClerck
Cell Rep, 2018-11-20;25(8):2177-2191.e7.
Species: Human
Sample Types: Cell Culture Supernates
-
Sera from Visceral Leishmaniasis Patients Display Oxidative Activity and Affect the TNF-? Production by Macrophages In Vitro
Authors: NM Soares, JN de Souza, TF Leal, EAG Reis, MS Miranda, WLC Dos Santos, MCA Teixeira
Biomed Res Int, 2017-11-05;2017(0):5861453.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel susceptibility locus in the IL12B region is associated with the pathophysiology of Takayasu arteritis through IL-12p40 and IL-12p70 production
Authors: T Nakajima, H Yoshifuji, M Shimizu, K Kitagori, K Murakami, R Nakashima, Y Imura, M Tanaka, K Ohmura, F Matsuda, C Terao, T Mimori
Arthritis Res. Ther., 2017-09-06;19(1):197.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages
Authors: AB Guimarães-, NC Rochael, F Oliveira, J Echevarria, EM Saraiva
Front Immunol, 2017-05-17;8(0):523.
Species: Human
Sample Types: Cell Culture Supernates
-
On the cytokine/chemokine network during Plasmodium vivax malaria: new insights to understand the disease
Authors: NS Hojo-Souza, DB Pereira, FS de Souza, TA de Oliveir, MS Cardoso, MS Tada, GM Zanini, DC Bartholome, RT Fujiwara, LL Bueno
Malar. J, 2017-01-24;16(1):42.
Species: Human
Sample Types: Plasma
-
High IFN-gamma and low SLPI mark severe asthma in mice and humans.
Authors: Raundhal M, Morse C, Khare A, Oriss T, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A
J Clin Invest, 2015-06-29;125(8):3037-50.
-
The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling.
Authors: Fleming B, Chandrasekaran P, Dillon L, Dalby E, Suresh R, Sarkar A, El-Sayed N, Mosser D
J Leukoc Biol, 2015-06-05;98(3):395-407.
Species: Human
Sample Types: Cell Culture Supernates
-
Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2.
Authors: Jaguin M, Fardel O, Lecureur V
PLoS ONE, 2015-02-24;10(2):e0116560.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of cytokines on spontaneous hepatitis B surface antigen seroconversion in chronic hepatitis B virus infection.
Authors: Wu J, Hsu H, Chiu Y, Chen H, Ni Y, Chang M
J Immunol, 2014-12-12;194(2):690-6.
Species: Human
Sample Types: Serum
-
Mycobacterium tuberculosis impairs dendritic cell functions through the serine hydrolase Hip1.
Authors: Madan-Lala R, Sia J, King R, Adekambi T, Monin L, Khader S, Pulendran B, Rengarajan J
J Immunol, 2014-03-21;192(9):4263-72.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR3 impairment in human newborns.
Authors: Slavica L, Nordstrom I, Karlsson M, Valadi H, Kacerovsky M, Jacobsson B, Eriksson K
J Leukoc Biol, 2013-07-30;94(5):1003-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of soluble vascular endothelial growth factor receptor-1 in human monocyte-derived mature dendritic cells contributes to their antiangiogenic property.
Authors: Kishuku M, Nishioka Y, Abe S, Kishi J, Ogino H, Aono Y, Azuma M, Kinoshita K, Batmunkh R, Rentsenhand B, Makino H, Ranjan P, Minakuchi K, Sone S
J. Immunol., 2009-12-15;183(12):8176-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.
Authors: Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G
Circulation, 2009-03-02;119(10):1424-32.
Species: Human
Sample Types: Plasma
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity.
Authors: Lauzon NM, Mian F, MacKenzie R, Ashkar AA
Cell. Immunol., 2006-10-17;241(2):102-12.
Species: Human
Sample Types: Cell Culture Supernates
-
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.
Authors: Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA
Am. J. Pathol., 2006-09-01;169(3):987-98.
Species: Human
Sample Types: Tissue Homogenates
-
A combination of E. coli DNA fragments and modified lipopolysaccharides as a cancer immunotherapy.
Authors: Cho YJ, Ahn BY, Lee NG, Lee DH, Kim DS
Vaccine, 2006-05-06;24(31):5862-71.
Species: Human
Sample Types: Cell Culture Supernates
-
The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation.
Authors: Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N
Blood, 2006-04-06;108(3):965-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing.
Authors: Anderson ST, Williams AJ, Brown JR, Newton SM, Simsova M, Nicol MP, Sebo P, Levin M, Wilkinson RJ, Wilkinson KA
Am. J. Respir. Crit. Care Med., 2006-02-02;173(9):1038-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists.
Authors: Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M
Eur. J. Immunol., 2005-08-01;35(8):2459-70.
Species: Human
Sample Types: Cell Culture Supernates
-
Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody.
Authors: Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G
J. Immunol., 2003-12-15;171(12):6466-77.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-12/IL-23 p40 DuoSet ELISA
Average Rating: 5 (Based on 3 Reviews)
Have you used Human IL-12/IL-23 p40 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
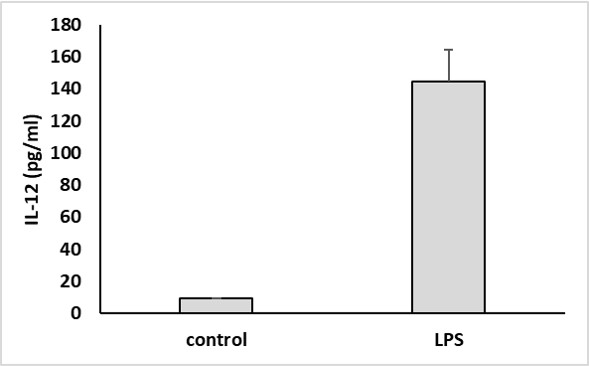
IL-12 secretion was measured in the supernatant of LPS-stimulated (100 ng/ml) cultured human dendritic cells after 18 hours.
Monocytes were stimulated with zymosan (25 μg/ml) for 24 hours. After 24 hours, IL-12 was assessed in the supernatant.