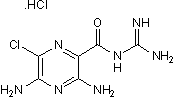
Amiloride hydrochloride
Chemical Name: 3,5-Diamino-N-(aminoiminomethyl)-6-chloropyrazinecarboxamide hydrochloride
Purity: ≥98%
Biological Activity
Amiloride hydrochloride is a Na+ channel blocker. Defines the I2A-amiloride sensitive and I2B-amiloride insensitive imidazoline binding Blocks TRPP3, acid sensing- (ASIC) and mechanogated membrane-ion channels, as well as the Na+/H+ exchanger. Also inhibits urokinase-type plasminogen activator (uPA); has no effect on tissue-type plasminogen activator.Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Background References
-
Evaluation of the role of nitric oxide in acid sensing ion channel mediated cell death.
Jetti et al.
Nitric Oxide, 2010;22:213 -
The pharmacology of mechanogated membrane ion channels.
Hamill and McBride
Pharmacol.Rev., 1996;48:231 -
Amiloride and its analogues as tools in the study of ion transport.
Kleyman et al.
J.Membr.Biol., 1988;105:1 -
A second generation of centrally acting antihypertensive agents act on putative I1-imidazoline receptors.
Ernsberger et al.
J.Cardiovasc.Pharmacol., 1992;20:S1 -
Inhibition of TRPP3 channel by amil. and analogs.
Dai et al.
Mol.Pharmacol., 2007;72:1576
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for Amiloride hydrochloride
The citations listed below are publications that use Tocris products. Selected citations for Amiloride hydrochloride include:
7 Citations: Showing 1 - 7
-
NCX1 Represents an Ionic Na+ Sensing Mechanism in Macrophages
Authors: Neubert Et al.
PloS Biol 2020;18
-
The Role of the Anion in Salt (NaCl) Detection by Mouse Taste Buds.
Authors: Roebber Et al.
J Neurosci 2019;39:6224
-
Osmotic induction of cyclooxygenase-2 in RPE cells: Stimulation of inflammasome activation.
Authors: Messerschmidt Et al.
Mol Vis 2019;25:329
-
Effects of the potassium-sparing diuretic amil. on chemotherapy response in canine osteosarcoma cells.
Authors: Poon Et al.
J Vet Intern Med 2018;33:800
-
Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension.
Authors: Barbaro
Cell Rep 2017;21(4):1009
-
Polycaprolactone/maltodextrin nanocarrier for intracellular drug delivery: formulation, uptake mechanism, internalization kinetics, and subcellular localization.
Authors: Korang-Yeboah Et al.
J Neurosci 2015;10:4763
-
Antinociceptive effects of amil. and benzamil in neuropathic pain model rats.
Authors: Jeong Et al.
Int J Nanomedicine 2013;28:1238
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for Amiloride hydrochloride
There are currently no reviews for this product. Be the first to review Amiloride hydrochloride and earn rewards!
Have you used Amiloride hydrochloride?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image