Human/Mouse/Rat HIF-1 alpha/HIF1A Antibody Summary
Arg575-Asn826
Accession # Q16665.1
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human HIF‑1 alpha /HIF1A by Western Blot. Western blot shows lysates of MCF-7 human breast cancer cell line untreated (-) or treated (+) with 150 µM CoCl2for 16 hours. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse/Rat HIF-1a/HIF1A Antigen-affinity Purified Polyclonal Antibody, followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). A specific band was detected for HIF-1a/HIF1A at approximately 120 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
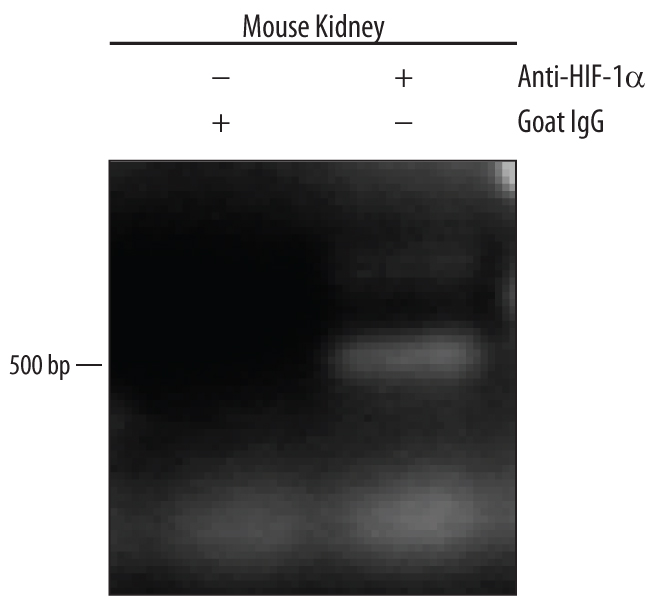
Detection of HIF‑1 alpha /HIF1A-regulated Genes by Chromatin Immunoprecipitation. Mouse primary kidney cells treated with 150 µM CoCl2 for overnight were fixed using formaldehyde, resuspended in lysis buffer, and sonicated to shear chromatin. HIF-1a/DNA complexes were immunoprecipitated using 5 µg Goat Anti-Human/Mouse/Rat HIF-1a/HIF1A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1935) or control antibody (Catalog # AB-108-C) for 15 minutes in an ultrasonic bath, followed by Biotinylated Anti-Goat IgG Secondary Antibody (Catalog # BAF109). Immunocomplexes were captured using 50 µL of MagCellect Streptavidin Ferrofluid (Catalog # MAG999) and DNA was purified using chelating resin solution. The epo promoter was detected by standard PCR.
 View Larger
View Larger
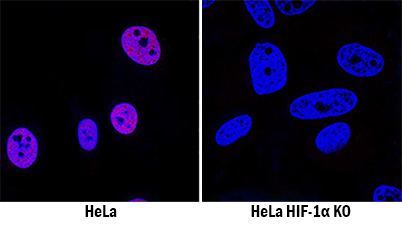
HIF‑1 alpha /HIF1A Specificity is Shown by Immunocytochemistry in Knockout Cell Line. HIF-1a/HIF1A was detected in immersion fixed HeLa human cervical epithelial carcinoma cell line treated with DFO but is not detected in HIF-1a/HIF1A knockout (KO) HeLa Human Cell Line cell line using Goat Anti-Human/Mouse/Rat HIF-1a/HIF1A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1935) at 0.3 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to nuclei. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
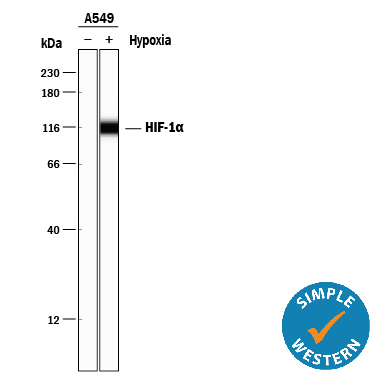
Detection of Human HIF‑1 alpha /HIF1A by Simple WesternTM. Simple Western lane view shows lysates of A549 human lung carcinoma cell line untreated (-) or treated (+) with Hypoxia (1% O2), loaded at 0.2 mg/mL. A specific band was detected for HIF-1a/HIF1A at approximately 115 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse/Rat HIF-1a/HIF1A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1935) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: HIF-1 alpha/HIF1A
The hypoxia-inducible transcription factor 1 alpha (HIF-1 alpha ) is the regulated member of the transcription factor heterodimer HIF-1. HIF-1 binds to hypoxia-response elements (HREs) in the promoters of many genes involved in adapting to an environment of insufficient oxygen or hypoxia. Hypoxic tissue environments occur in vascular and pulmonary diseases as well as cancer, which illustrates the broad impact of gene regulation by HIF-1 alpha.
Product Datasheets
Citations for Human/Mouse/Rat HIF-1 alpha/HIF1A Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
47
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
HIF-1alpha regulates CD55 expression in airway epithelium.
Authors: Pandya PH, Wilkes DS.
Am J Respir Cell Mol Biol
-
Local lung hypoxia determines epithelial fate decisions during alveolar regeneration
Authors: Ying Xi, Thomas Kim, Alexis N. Brumwell, Ian H. Driver, Ying Wei, Victor Tan et al.
Nature Cell Biology
-
MTA1 aggravates experimental colitis in mice by promoting transcription factor HIF1A and up-regulating AQP4 expression
Authors: Ping Li, Dong-Ping Shi, Tao Jin, Dong Tang, Wei Wang, Liu-Hua Wang
Cell Death Discovery
-
LOX-1 mediates inflammatory activation of microglial cells through the p38-MAPK/NF-kappa B pathways under hypoxic-ischemic conditions
Authors: Yoshinori Aoki, Hongmei Dai, Fumika Furuta, Tomohisa Akamatsu, Takuya Oshima, Naoto Takahashi et al.
Cell Communication and Signaling
-
Epigenetic Reprogramming of Cancer-Associated Fibroblasts Deregulates Glucose Metabolism and Facilitates Progression of Breast Cancer
Authors: Becker LM, O'Connell JT, Vo AP et al.
Cell Rep
-
Hypoxia on the Expression of Hepatoma Upregulated Protein in Prostate Cancer Cells
Authors: Ingrid Espinoza, Marcelo J. Sakiyama, Tangeng Ma, Logan Fair, Xinchun Zhou, Mohamed Hassan et al.
Frontiers in Oncology
-
HRAS as a potential therapeutic target of salirasib RAS inhibitor in bladder cancer
Authors: Satoshi Sugita, Hideki Enokida, Hirofumi Yoshino, Kazutaka Miyamoto, Masaya Yonemori, Takashi Sakaguchi et al.
International Journal of Oncology
-
A network of RNA-binding proteins controls translation efficiency to activate anaerobic metabolism
Authors: Ho JJD, Balukoff NC, Theodoridis PR et al.
Nat Commun
-
Human TM9SF4 Is a New Gene Down-Regulated by Hypoxia and Involved in Cell Adhesion of Leukemic Cells
Authors: Rosa Paolillo, Isabella Spinello, Maria Teresa Quaranta, Luca Pasquini, Elvira Pelosi, Francesco Lo Coco et al.
PLOS ONE
-
Hypoxia-induced HIF1 alpha targets in melanocytes reveal a molecular profile associated with poor melanoma prognosis
Authors: Stacie K. Loftus, Laura L. Baxter, Julia C. Cronin, Temesgen D. Fufa, William J. NISC Comparative Sequencing Program, William J. NISC Comparative Sequencing Program
Pigment Cell & Melanoma Research
-
Increased Ascorbate Content of Glioblastoma Is Associated With a Suppressed Hypoxic Response and Improved Patient Survival
Authors: Eleanor R. Burgess, Rebekah L. I. Crake, Elisabeth Phillips, Helen R. Morrin, Janice A. Royds, Tania L. Slatter et al.
Frontiers in Oncology
-
A HIF1a-Dependent Pro-Oxidant State Disrupts Synaptic Plasticity and Impairs Spatial Memory in Response to Intermittent Hypoxia.
Authors: Arias-Cavieres A, Khuu M A et al.
Eneuro
Species: Mouse
Sample Types:
Applications: Simple Western -
Lactate limits CNS autoimmunity by stabilizing HIF-1? in dendritic cells
Authors: Sanmarco, LM;Rone, JM;Polonio, CM;Fernandez Lahore, G;Giovannoni, F;Ferrara, K;Gutierrez-Vazquez, C;Li, N;Sokolovska, A;Plasencia, A;Faust Akl, C;Nanda, P;Heck, ES;Li, Z;Lee, HG;Chao, CC;Rejano-Gordillo, CM;Fonseca-Castro, PH;Illouz, T;Linnerbauer, M;Kenison, JE;Barilla, RM;Farrenkopf, D;Stevens, NA;Piester, G;Chung, EN;Dailey, L;Kuchroo, VK;Hava, D;Wheeler, MA;Clish, C;Nowarski, R;Balsa, E;Lora, JM;Quintana, FJ;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC/IF -
Partial Mural Cell Ablation Disrupts Coronary Vasculature Integrity and Induces Systolic Dysfunction
Authors: Cornuault, L;Hérion, FX;Bourguignon, C;Rouault, P;Foussard, N;Alzieu, P;Chapouly, C;Gadeau, AP;Couffinhal, T;Renault, MA;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Engineered probiotics limit CNS autoimmunity by stabilizing HIF-1alpha in dendritic cells
Authors: LM Sanmarco, JM Rone, CM Polonio, F Giovannoni, GF Lahore, K Ferrara, C Gutierrez-, N Li, A Sokolovska, A Plasencia, CF Akl, P Nanda, ES Heck, Z Li, HG Lee, CC Chao, CM Rejano-Gor, PH Fonseca-Ca, T Illouz, M Linnerbaue, JE Kenison, RM Barilla, D Farrenkopf, G Piester, L Dailey, VK Kuchroo, D Hava, MA Wheeler, C Clish, R Nowarski, E Balsa, JM Lora, FJ Quintana
bioRxiv : the preprint server for biology, 2023-03-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Extreme Hypoxia Causing Brady-Arrythmias During Apnea in Elite Breath-Hold Divers
Authors: T Kjeld, AB Isbrand, K Linnet, B Zerahn, J Højberg, EG Hansen, LC Gormsen, J Bejder, T Krag, J Vissing, HE Bøtker, HC Arendrup
Frontiers in Physiology, 2021-12-03;12(0):712573.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
FLI1 mediates the selective expression of hypoxia-inducible factor-1 target genes in endothelial cells under hypoxic conditions
Authors: G Zeng, T Wang, J Zhang, YJ Kang, L Feng
FEBS Open Bio, 2021-06-08;0(0):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
LncRNA-SNHG6 promotes the progression of hepatocellular carcinoma by targeting miR-6509-5p and HIF1A
Authors: X Fan, Z Zhao, J Song, D Zhang, F Wu, J Tu, M Xu, J Ji
Cancer Cell International, 2021-03-04;21(1):150.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Reciprocal Interaction between Vascular Filopodia and Neural Stem Cells Shapes Neurogenesis in the Ventral Telencephalon
Authors: B Di Marco, EE Crouch, B Shah, C Duman, MF Paredes, C Ruiz de Al, EJ Huang, J Alfonso
Cell Rep, 2020-10-13;33(2):108256.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CCR2 of Tumor Microenvironmental Cells Is a Relevant Modulator of Glioma Biology
Authors: M Felsenstei, A Blank, AD Bungert, A Mueller, A Ghori, I Kremenetsk, O Rung, T Broggini, K Turkowski, L Scherschin, J Raggatz, P Vajkoczy, S Brandenbur
Cancers (Basel), 2020-07-13;12(7):.
Species: Mouse
Sample Types: Brain Section
Applications: IHC-F -
The critical role of MMP14 in adipose tissue remodeling during obesity
Authors: X Li, Y Zhao, C Chen, L Yang, HH Lee, Z Wang, N Zhang, MG Kolonin, Z An, X Ge, PE Scherer, K Sun
Mol. Cell. Biol., 2020-03-30;0(0):.
Species: Human
Sample Types: Chromatin
Applications: ChIP (chromatin immunoprecipit -
HIF prolyl hydroxylase inhibition protects skeletal muscle from eccentric contraction-induced injury
Authors: AN Billin, SE Honeycutt, AV McDougal, JP Kerr, Z Chen, JM Freudenber, DK Rajpal, G Luo, HF Kramer, RS Geske, F Fang, B Yao, RV Clark, J Lepore, A Cobitz, R Miller, K Nosaka, AC Hinken, AJ Russell
Skelet Muscle, 2018-11-13;8(1):35.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Co-Expression of VEGF and IL-6 Family Cytokines is Associated with Decreased Survival in HER2 Negative Breast Cancer Patients: Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion
Authors: K Tawara, H Scott, J Emathinger, A Ide, R Fox, D Greiner, D LaJoie, D Hedeen, M Nandakumar, AJ Oler, R Holzer, C Jorcyk
Transl Oncol, 2018-11-12;12(2):245-255.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
PRKAA1/AMPK?1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis
Authors: Q Yang, J Xu, Q Ma, Z Liu, V Sudhahar, Y Cao, L Wang, X Zeng, Y Zhou, M Zhang, Y Xu, Y Wang, NL Weintraub, C Zhang, T Fukai, C Wu, L Huang, Z Han, T Wang, DJ Fulton, M Hong, Y Huo
Nat Commun, 2018-11-07;9(1):4667.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis
Authors: Z Liu, S Yan, J Wang, Y Xu, Y Wang, S Zhang, X Xu, Q Yang, X Zeng, Y Zhou, X Gu, S Lu, Z Fu, DJ Fulton, NL Weintraub, RB Caldwell, W Zhang, C Wu, XL Liu, JF Chen, A Ahmad, I Kaddour-Dj, M Al-Shabraw, Q Li, X Jiang, Y Sun, A Sodhi, L Smith, M Hong, Y Huo
Nat Commun, 2017-09-19;8(1):584.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Forkhead Box O3 (FoxO3) Regulates Kidney Tubular Autophagy Following Urinary Tract Obstruction
Authors: L Li, R Zviti, C Ha, ZV Wang, JA Hill, F Lin
J. Biol. Chem., 2017-07-13;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Metabolic reprogramming is associated with flavopiridol resistance in prostate cancer DU145 cells
Authors: X Li, J Lu, Q Kan, X Li, Q Fan, Y Li, R Huang, A Slipicevic, HP Dong, L Eide, J Wang, H Zhang, V Berge, MA Goscinski, G Kvalheim, JM Nesland, Z Suo
Sci Rep, 2017-07-11;7(1):5081.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Motor neurons control blood vessel patterning in the developing spinal cord
Authors: P Himmels, I Paredes, H Adler, A Karakatsan, R Luck, HH Marti, O Ermakova, E Rempel, ET Stoeckli, C Ruiz de Al
Nat Commun, 2017-03-06;8(0):14583.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
HEREGULIN/ErbB3 SIGNALING ENHANCES CXCR4-DRIVEN Rac1 ACTIVATION AND BREAST CANCER CELL MOTILITY VIA HIF-1?
Mol Cell Biol, 2016-07-14;0(0):.
Species: Human
Sample Types: Chromatin
Applications: ChIP -
Identification of ?-Dystrobrevin as a Direct Target of miR-143: Involvement in Early Stages of Neural Differentiation
PLoS ONE, 2016-05-25;11(5):e0156325.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Ascorbate availability affects tumor implantation-take rate and increases tumor rejection in Gulo(-/-) mice
Authors: Gabi U Dachs
Hypoxia (Auckl), 2016-04-08;4(0):41-52.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Warburg-like Glycolysis and Lactate Shuttle in Mouse Decidua during Early Pregnancy.
Authors: Zuo R, Gu X, Qi Q, Wang T, Zhao X, Liu J, Yang Z
J Biol Chem, 2015-07-15;290(35):21280-91.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
High Glucose Up-regulates ADAM17 through HIF-1alpha in Mesangial Cells.
Authors: Li R, Uttarwar L, Gao B, Charbonneau M, Shi Y, Chan J, Dubois C, Krepinsky J
J Biol Chem, 2015-07-14;290(35):21603-14.
Species: Rat
Sample Types: Cell Lysates
Applications: ChIP -
Stress-resistant Translation of Cathepsin L mRNA in Breast Cancer Progression.
Authors: Tholen M, Wolanski J, Stolze B, Chiabudini M, Gajda M, Bronsert P, Stickeler E, Rospert S, Reinheckel T
J Biol Chem, 2015-05-08;290(25):15758-69.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Myeloid cell leukemia-1 (Mc1-1) is a candidate target gene of hypoxia-inducible factor-1 (HIF-1) in the testis.
Authors: Palladino M, Shah A, Tyson R, Horvath J, Dugan C, Karpodinis M
Reprod Biol Endocrinol, 2012-12-05;10(0):104.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ChIP, Direct ELISA -
The hypoxic microenvironment upgrades stem-like properties of ovarian cancer cells.
Authors: Liang D, Ma Y, Liu J, Trope CG, Holm R, Nesland JM, Suo Z
BMC Cancer, 2012-05-29;12(0):201.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
HIF-1-mediated up-regulation of cardiotrophin-1 is involved in the survival response of cardiomyocytes to hypoxia.
Authors: Robador PA, San Jose G, Rodriguez C, Guadall A, Moreno MU, Beaumont J, Fortuno A, Diez J, Martinez-Gonzalez J, Zalba G
Cardiovasc. Res., 2011-07-19;92(2):247-55.
Species: Human
Sample Types: Cell Lysates
Applications: ChIP -
Development of an in vitro model of myotube ischemia.
Authors: Joshi D, Patel H, Baker DM, Shiwen X, Abraham DJ, Tsui JC
Lab. Invest., 2011-05-23;91(8):1241-52.
Species: Mouse
Sample Types: Whole Cells
Applications: Western Blot -
HER-2 signaling, acquisition of growth factor independence, and regulation of biological networks associated with cell transformation.
Authors: Bollig-Fischer A, Dziubinski M, Boyer A, Haddad R, Giroux CN, Ethier SP
Cancer Res., 2010-08-24;70(20):7862-73.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes.
Authors: Wang B, Wood IS, Trayhurn P
J. Endocrinol., 2008-05-07;198(1):127-34.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The new tumor-suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: involvement in myeloma-induced angiogenesis.
Authors: Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L, Ravanetti L, Bonomini S, Ferrari L, Miranda C, Ladetto M, Neri TM, Neri A, Greco A, Mangoni M, Bonati A, Rizzoli V, Giuliani N
Blood, 2007-09-11;110(13):4464-75.
Species: Human
Sample Types: Nuclear Extract
Applications: Western Blot -
Amylin deposition activates HIF1 alpha and 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) signaling in failing hearts of non-human primates
Authors: Miao Liu, Nan Li, Chun Qu, Yilin Gao, Lijie Wu, Liangbiao George Hu
Communications Biology
-
Astrocyte HIF-2α supports learning in a passive avoidance paradigm under hypoxic stress
Authors: Cindy V Leiton, Elyssa Chen, Alissa Cutrone, Kristy Conn, Kennelia Mellanson, Dania M Malik et al.
Hypoxia (Auckl)
-
Effects of lipopolysaccharide-induced inflammation on hypoxia and inflammatory gene expression pathways of the rat testis
Authors: Michael A. Palladino, Genevieve A. Fasano, Dharm Patel, Christine Dugan, Marie London
Basic and Clinical Andrology
-
Galectin-1 suppression delineates a new strategy to inhibit myeloma-induced angiogenesis and tumoral growth in vivo
Authors: P Storti, V Marchica, I Airoldi, G Donofrio, E Fiorini, V Ferri et al.
Leukemia
-
A translational program that suppresses metabolism to shield the genome
Authors: NC Balukoff, JJD Ho, PR Theodoridi, M Wang, M Bokros, LM Llanio, JR Krieger, JH Schatz, S Lee
Nat Commun, 2020-11-13;11(1):5755.
-
New panel of biomarkers to discriminate between amelanotic and melanotic metastatic melanoma
Authors: Ioana V. Militaru, Alina Adriana Rus, Cristian V.A. Munteanu, Georgiana Manica, Stefana M. Petrescu
Frontiers in Oncology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat HIF-1 alpha/HIF1A Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Human/Mouse/Rat HIF-1 alpha/HIF1A Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:





