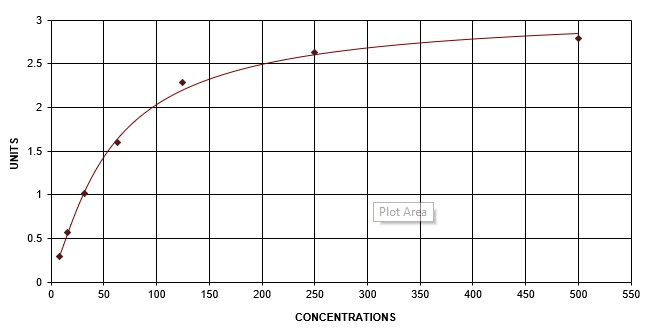
Human CCL17/TARC DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human CCL17/TARC. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL17/TARC
CCL17/TARC is a chemokine that interacts with CCR4 and CCR8 to induce the chemoattraction of activated Th2 cells, basophils, and NK cells. It is produced by thymic dendritic cells, lymph node dendritic cells, monocytes, CD4+ T cells, keratinocytes, fibroblasts, bronchial epithelial cells, and Reed-Sternberg cells. CCL17 promotes Th2 cell recruitment to sites of inflammation and induces platelet aggregation and degranulation.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human CCL17/TARC DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
22
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The IL-4/13-induced production of M2 chemokines by human lung macrophages is enhanced by adenosine and PGE2
Authors: Brollo, M;Salvator, H;Grassin-Delyle, S;Glorion, M;Descamps, D;Buenestado, A;Naline, E;Tenor, H;Tiotiu, A;Devillier, P;
International immunopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
Bronchoalveolar cytokine profile differentiates Pulmonary Langerhans cell histiocytosis patients from other smoking-related interstitial lung diseases
Authors: Barril, S;Acebo, P;Millan-Billi, P;Luque, A;Sibila, O;Tarín, C;Tazi, A;Castillo, D;Hortelano, S;
Respiratory research
Species: Human
Sample Types: BALF
-
Human interleukin-12? and EBI3 are cytokines with anti-inflammatory functions
Authors: Hildenbrand, K;Bohnacker, S;Menon, PR;Kerle, A;Prodjinotho, UF;Hartung, F;Strasser, PC;Catici, DAM;Rührnö beta l, F;Haslbeck, M;Schumann, K;Müller, SI;da Costa, CP;Esser-von Bieren, J;Feige, MJ;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro assessment of the anti-inflammatory and skin-moisturizing effects of Filipendula palmata (Pall.) Maxim. On human keratinocytes and identification of its bioactive phytochemicals
Authors: XJ Mi, JK Kim, S Lee, SK Moon, YJ Kim, H Kim
Journal of ethnopharmacology, 2022-07-07;296(0):115523.
Species: Human
Sample Types: Cell Culture Supernates
-
Serological profiling reveals hsa-miR-451a as a possible biomarker of anaphylaxis
Authors: W Francuzik, K Pažur, M Dalke, S Dölle-Bier, M Babina, M Worm
JCI Insight, 2022-04-08;0(0):.
Species: Human
Sample Types: Serum
-
Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up
Authors: B Grüner, L Peters, A Hillenbran, P Vo beta berg, J Schweiker, EG Rollmann, LH Rodriguez, J Blumhardt, S Burkert, P Kern, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-02-02;16(2):e0010099.
Species: Human
Sample Types: Cell Culture Supernates
-
Gomisin M2 alleviates psoriasis?like skin inflammation by inhibiting inflammatory signaling pathways
Authors: N Kim, S Lee, J Kang, TK Kwon, D Khang, SH Kim
Molecular Medicine Reports, 2021-10-19;24(6):.
Species: Human
Sample Types: Serum
-
Skullcapflavone II Suppresses TNF-alpha/IFN-gamma-Induced TARC, MDC, and CTSS Production in HaCaT Cells
Authors: H Lee, DH Lee, JH Oh, JH Chung
International Journal of Molecular Sciences, 2021-06-16;22(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation
Authors: GK Vasileiadi, AC Lundell, Y Zhang, K Andersson, I Gjertsson, A Rudin, C Maglio
Biomolecules, 2021-02-21;11(2):.
Species: Human
Sample Types: Plasma
-
Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism
Authors: M Mazzoni, G Mauro, M Erreni, P Romeo, E Minna, MG Vizioli, C Belgiovine, MG Rizzetti, S Pagliardin, R Avigni, MC Anania, P Allavena, MG Borrello, A Greco
J. Exp. Clin. Cancer Res., 2019-05-21;38(1):208.
Species: Human
Sample Types: Cell Culture Supernates
-
Rhododendron album Blume extract inhibits TNF-?/IFN-?-induced chemokine production via blockade of NF-?B and JAK/STAT activation in human epidermal keratinocytes
Authors: JW Park, HS Lee, Y Lim, JH Paik, OK Kwon, JH Kim, I Paryanto, P Yunianto, S Choi, SR Oh, KS Ahn
Int. J. Mol. Med., 2018-03-09;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Unbiased Quantitative Proteomics Reveals a Crucial Role of the Allergen Context for the Activation of Human Dendritic Cells
Authors: L Strasser, HH Dang, H Schwarz, C Asam, F Ferreira, J Horejs-Hoe, CG Huber
Sci Rep, 2017-11-30;7(1):16638.
Species: Human
Sample Types: Cell Culture Supernates
-
Forsythia suspensa Suppresses House Dust Mite Extract-Induced Atopic Dermatitis in NC/Nga Mice
PLoS ONE, 2016-12-09;11(12):e0167687.
Species: Human
Sample Types: Cell Culture Supernates
-
Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2.
Authors: Jaguin M, Fardel O, Lecureur V
PLoS ONE, 2015-02-24;10(2):e0116560.
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis.
Authors: Schupp J, Binder H, Jager B, Cillis G, Zissel G, Muller-Quernheim J, Prasse A
PLoS ONE, 2015-01-15;10(1):e0116775.
Species: Human
Sample Types: BALF
-
Human B cells induce dendritic cell maturation and favour Th2 polarization by inducing OX-40 ligand.
Authors: Maddur M, Sharma M, Hegde P, Stephen-Victor E, Pulendran B, Kaveri S, Bayry J
Nat Commun, 2014-06-09;5(0):4092.
Species: Human
Sample Types: Cell Culture Supernates
-
Marked and persistent eosinophilia in the absence of clinical manifestations.
Authors: Chen Y, Khoury P, Ware J, Holland-Thomas N, Stoddard J, Gurprasad S, Waldner A, Klion A
J Allergy Clin Immunol, 2013-08-26;133(4):1195-202.
Species: Human
Sample Types: Serum
-
Identification of a New Phenotype of Tolerogenic Human Dendritic Cells Induced by Fungal Proteases from Aspergillus oryzae.
Authors: Zimmer A, Luce S, Gaignier F, Nony E, Naveau M, Biola-Vidamment A, Pallardy M, Van Overtvelt L, Mascarell L, Moingeon P
J. Immunol., 2011-03-02;186(7):3966-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Anemia in Hodgkin's lymphoma: the role of interleukin-6 and hepcidin.
Authors: Hohaus S, Massini G, Giachelia M, Vannata B, Bozzoli V, Cuccaro A, D'Alo' F, Larocca LM, Raymakers RA, Swinkels DW, Voso MT, Leone G
J. Clin. Oncol., 2010-04-20;28(15):2538-43.
Species: Human
Sample Types: Plasma
-
Pathogenesis of cBFL in common with IPF? Correlation of IP-10/TARC ratio with histological patterns.
Authors: Kishi M, Miyazaki Y, Jinta T, Furusawa H, Ohtani Y, Inase N, Yoshizawa Y
Thorax, 2008-02-14;63(9):810-6.
Species: Human
Sample Types: Serum
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17.
Authors: Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW
J. Immunol., 2007-08-01;179(3):1648-58.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human CCL17/TARC DuoSet ELISA
Average Rating: 5 (Based on 2 Reviews)
Have you used Human CCL17/TARC DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥1250 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: